Abstract
Duodenal diverticula are detected in up to 27% of patients undergoing upper gastrointestinal tract evaluation with periampullary diverticula (PAD) being the most common type. Although PAD usually do not cause symptoms, it can serve as a source of obstructive jaundice even when choledocholithiasis or tumor is not present. This duodenal diverticulum obstructive jaundice syndrome is called Lemmel's syndrome. An 81-yr-old woman came to the emergency room with obstructive jaundice and cholangitis. Abdominal CT scan revealed stony opacity on distal CBD with CBD dilatation. ERCP was performed to remove the stone. However, the stone was not located in the CBD but rather inside the PAD. After removal of the enterolith within the PAD, all her symptoms resolved. Recognition of this condition is important since misdiagnosis could lead to mismanagement and therapeutic delay. Lemmel's syndrome should always be included as one of the differential diagnosis of obstructive jaundice when PAD are present.
Periampullary diverticula (PAD) refer to extraluminal outpouchings of duodenal mucosa that develop within the radius of 2 to 3 cm from the ampulla of Vater (1). PAD are largely asymptomatic but they sometimes can cause both pancreaticobiliary and non-pancreaticobiliary complications. Rarely, obstructive jaundice can develop secondary to PAD in the absence of choledocholithiasis or tumor and is termed Lemmel's syndrome (2). Recently, the authors experienced an unusual case of abdominal pain and obstructive jaundice due to extrinsic compression of mid common bile duct (CBD) by distended PAD filled with pus-like material as a result of impacted intradiverticular enterolith at the PAD orifice. Herein, we report a case Lemmel's syndrome that was successfully managed endoscopically.
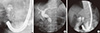
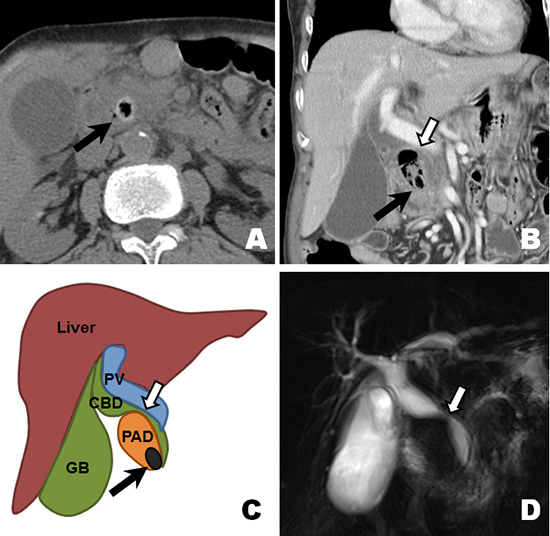
An 81-yr-old woman presented to the emergency department on August 3, 2012 with nausea, vomiting, fever (38.4℃), and diffuse abdominal pain of 4 days' duration. She had undergone subtotal gastrectomy with billroth II anastomosis due to peptic ulcer perforation 10 yr ago. On physical examination, there was tenderness on her right upper quadrant but Murphy's sign was equivocal. Her white blood count was increased to 11,170/µL (neutrophil 83.0%) and CRP was elevated to 2.392 mg/dL. The results of her liver function test were as follows: total bilirubin, 2.37 mg/dL; aspartate aminotransferase, 88 IU/L; alanine aminotransferase, 96 IU/L; alkaline phosphatase, 349 IU/L; and γ-glutamyl transpeptidase, 571 IU/L. To evaluate the cause of diffuse abdominal pain with liver enzyme elevation in a cholestatic pattern, abdominal CT scan was taken. The axial images of the CT scan demonstrated distal CBD stone with upstream bile duct dilatation (Fig. 1A). However, on coronal reconstructed images, the stone was not located within the bile duct but inside the PAD and the distended diverticulum was compressing the mid CBD (Fig. 1B, 1C). Magnetic resonance cholangiopancreatography (MRCP) also revealed mid CBD compression and absence of choledocholithiasis (Fig. 1D). These findings were confirmed on endoscopic retrograde cholangiopancreatography (ERCP) which showed normal biliary orifice (Fig. 2A) with impacted dark brown pigment stone (henceforth enterolith) at the PAD orifice (Fig. 2B). When the enterolith was pushed into the diverticulum by cannulation catheter and contrast dye was injected (Fig. 2C, 3A), old blood clots and pus-like fluid gushed out from the opening (Fig. 2D). Biliary cannulation combined with endoscopic sphincterotomy (EST) was also performed to explore the CBD for other possible causes of obstructive jaundice but no stone, stricture or obstruction by tumor could be found. On endoscopic nasobiliary drainage (ENBD) tubogram, stenosis at mid CBD was also shown to be resolved, likely because the PAD had been decompressed (Fig. 3B). After confirming that no other pathology was present, intradiverticular enterolith was crushed and removed by Dormia basket on the following day (Fig. 2E, F). After the procedure, the patient no longer complained of abdominal pain, her liver enzyme was normalized, and she was discharged without any complication.
She had been well until 6 months after enterolith removal, when the patient visited the outpatient clinic with vague abdominal discomfort. Laboratory examination only revealed slightly increased total bilirubin to 1.96 mg/dL, but a large CBD stone was found on abdominal CT scan (Fig. 4A). When the CBD stone was removed by ERCP, the stone proved to be brown pigment sludge stone that typically forms in the presence of ascending infection (Fig. 4B, C). PAD at this time was neither distended nor filled with enterolith. Follow-up ERCP was performed again after 6 months but there was no recurrence of CBD stone or enterolith. She remains clinically and radiographically disease free after the last procedure.
Diverticula of the gastrointestinal tract are outpouchings of all or part of the intestinal wall which can occur anywhere throughout the alimentary tract. The most common site of gastrointestinal diverticula is colon followed by duodenum, which was first described by Chomel in 1710 (3). The detection rate of duodenal diverticula ranges from 1% to 27% depending on the diagnostic modalities used and the average age at the time of diagnosis (1). Among duodenal diverticula, PAD is the most common type comprising about 70% to 75% of all duodenal diverticula (1).
Most PAD are asymptomatic but complications can occur in about 5% of cases and they include bleeding, perforation, diverticulitis, pancreatitis, choledocholithiasis, cholangitis, jaundice, enterolith or bezoar formation, intestinal obstruction, etc. Among these complications, hepatocholangiopancreatic disease can seldomly occur in the absence of choledocholithiasis and is termed Lemmel's syndrome (2). Pathologic mechanisms through which Lemmel's syndrome is thought to occur include the following. First, diverticulitis or direct mechanical irritation of PAD may cause chronic inflammation of ampulla and lead to chronic fibrosis of papilla (papillitis chronica fibrosa) (4). Second, PAD many cause dysfunction in the sphincter of Oddi (5). Third, distal CBD or ampulla can be directly compressed mechanically by PAD that is usually filled with enterolith or bezoar (6, 7). In our case, enterolith that formed within the PAD did not directly compress the distal CBD but it obstructed the PAD orifice instead. This obstruction combined with inflammation of the diverticulum and collection of pus-like material within the obstructed PAD seems to have expanded the PAD with resultant extrinsic compression of mid CBD (Fig. 1). This was quite different from previous cases in which compression occurred at distal CBD. PAD normally have a relatively wide orifice. Therefore, the enterolith, bezoar, or food material within the PAD is frequently evacuated and thus, the symptom could be intermittent. However, PAD in our case had a narrow opening, likely due to repeated inflammation of the PAD, and this seems to have hindered the clearance of entrapped enterolith out into the duodenal lumen.
Enterolith formation within the duodenal diverticula is known to be facilitated in the static environment such as a blind loop after gastrectomy or proximal portion of stricture formed by Crohn's disease or tuberculosis (8). In our case, blind loop created by Billroth II anastomosis seems to have provided a static environment favoring enterolith formation within the PAD. During enterolith removal, CBD was also explored to search for other possible source of obstructive jaundice such as CBD stone since primary biliary stone is known to occur more frequently in the presence of PAD (9, 10). One possible mechanism behind increased occurrence of primary CBD stone in these patients involves colonization and overgrowth of β-glucuronidase producing bacteria within the PAD that spread into the bile duct, which in turn leads to deconjugation of bilirubin glucuronides and eventually results in precipitation of calcium bilirubinate gallstones (11). Although CBD was explored in our case, no other etiology of obstructive jaundice could be identified other than extrinsic compression by distended PAD. However, primary bile duct stone newly developed on follow-up ERCP performed 6 months later (Fig. 4). The most plausible explanation is that EST performed during CBD exploration at the time of enterolith removal has permitted the occurrence of ascending infection with resultant brown pigment stone formation.
Diagnosing Lemmel's syndrome could be challenging, but being aware of this condition is important to avoid mismanagement and it begins with identification of PAD. PAD are best demonstrated using a side-viewing endoscope during ERCP. On CT scan and MRCP, PAD appear as thin-walled cavitary lesions situated on the medial wall of the duodenum 2nd portion that typically contain gas. However, PAD are sometimes filled with fluid and can frequently be mistaken for pancreatic pseudocyst, pancreatic abscess, cystic neoplasm in the pancreas head or even metastatic lymph node (12, 13). Therefore, high index of suspicion is mandatory to arrive at a correct diagnosis in these patients. In our case, enterolith within the PAD on axial images was at first mistaken for distal CBD stone due to its distal location combined with upstream dilatation of the bile duct (Fig. 1A). However, upon careful scrutinization of the coronal reconstructed images, it became evident that the stone was located within the PAD (Fig. 1B, C) which was eventually confirmed by ERCP (Fig. 2).
Treatment is generally not recommended in asymptomatic patients or would be conservative management in pauci-symptomatic patients. Nevertheless, since most patients with Lemmel's syndrome present with symptoms related to biliary obstruction (i.e. jaundice, abdominal pain, and cholangitis) as a result of extrinsic compression of CBD, some form of treatment is advocated. Therapeutic options in this situation run the gamut from endoscopic extraction of entrapped material, extracorporeal shock wave lithotripsy to surgery (diverticulectomy or biliodigestive anastomosis) (7, 14, 15). The patient in our case was also successfully treated endoscopically by fragmenting and removing enterolith using a Dormia basket. It should be kept in mind that not all forms of Lemmel's syndrome are caused by extrinsic compression of CBD by bulging PAD. If the underlying mechanism of Lemmel's syndrome is likely to be due to papillitis chronica fibrosa or sphincter of Oddi dysfunction as mentioned above, the simplest and the most appropriate management would be to perform EST (16).
In conclusion, Lemmel's syndrome is a rare cause of obstructive jaundice that should be included in the differential diagnosis of biliary obstruction when PAD is present. Maintaining a high index of suspicion is imperative to establish an accurate diagnosis since it can mimic other cystic or solid lesions around the pancreas head. Symptomatic patient can be successfully managed endoscopically in many instances but recourse to surgical management would be necessary in selected cases.
Figures and Tables
Fig. 1
Computed tomography (CT) and magnetic resonance cholangiopancreatography (MRCP) findings of the biliary tract. On axial CT scan, a high attenuated stone density with internal air (black arrow) is seen on distal common bile duct (CBD) (A). However, coronal reconstructed image shows that the stone (black arrow) is not located in the CBD but within the periampullary diverticulum that is filled with air and debris along with mid CBD stricture (white arrow) (B). This finding is depicted in line art to better delineate the anatomical relationship (C). Absence of CBD stone and mid CBD stricture is also demonstrated on MRCP image (D).

Fig. 2
Endoscopic retrograde cholangiopancreatographic findings of enterolith within the periampullary diverticulum (PAD) and its removal. A dark brown pigment stone (white arrow) is seen impacted at the orifice of the PAD (A, B). When the stone is pushed upward into the PAD (C), old blood clots is seen gushing out from the PAD orifice (D). The enterolith within the PAD is being fragmented and removed with Dormia basket (E, F).
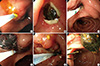
Fig. 3
Fluoroscopic images. When dye is injected into the PAD, an ovoid shaped filling defect (white arrow) can be seen (A). Endoscopic nasobiliary drainage tubogram obtained after decompression of the PAD demonstrates resolved extrinsic compression (B). After completion of enterolith removal, filling defect is no longer seen within the PAD (C).
References
1. Lobo DN, Balfour TW, Iftikhar SY, Rowlands BJ. Periampullary diverticula and pancreaticobiliary disease. Br J Surg. 1999; 86:588–597.
2. Lemmel G. Die Klinische Bedeutung der Duodenal Divertikel. Arch Verdauungskrht. 1934; 46:59–70.
3. Chomel JB. Report of a case of duodenal diverticulum containing gallstones. Histoire Acad R Sci Paris. 1710; 48–50.
4. Manabe T, Yu GS. Duodenal diverticulum causing intermittent-persistent cholestasis: associated with papillitis chronica fibrosa. N Y State J Med. 1977; 77:2132–2136.
5. Tomita R, Tanjoh K. Endoscopic manometry of the sphincter of Oddi in patients with Lemmel's syndrome. Surg Today. 1998; 28:258–261.
6. Rouet J, Gaujoux S, Ronot M, Palazzo M, Cauchy F, Vilgrain V, Belghiti J, O'Toole D, Sauvanet A. Lemmel's syndrome as a rare cause of obstructive jaundice. Clin Res Hepatol Gastroenterol. 2012; 36:628–631.
7. Nishida K, Kato M, Higashijima M, Takagi K, Akashi R. A case of Lemmel's syndrome caused by a large diverticular enterolith at the peripapillary portion of the duodenum. Nihon Ronen Igakkai Zasshi. 1995; 32:825–829.
8. Shocket E, Simon SA. Small bowel obstruction due to enterolith (bezoar) formed in a duodenal diverticulum: a case report and review of the literature. Am J Gastroenterol. 1982; 77:621–624.
9. Tham TC, Kelly M. Association of periampullary duodenal diverticula with bile duct stones and with technical success of endoscopic retrograde cholangiopancreatography. Endoscopy. 2004; 36:1050–1053.
10. Lee JH, Lee JS, Kim HW, Bae SM, Kim SH, Kang DH, Song CS, Song GA, Cho M, Yang US. Association of periampullary diverticula with primary choledocholithiasis. Korean J Gastroenterol. 1999; 33:252–257.
11. Skar V, Skar AG, Bratlie J, Osnes M. Beta-glucuronidase activity in the bile of gallstone patients both with and without duodenal diverticula. Scand J Gastroenterol. 1989; 24:205–212.
12. Macari M, Lazarus D, Israel G, Megibow A. Duodenal diverticula mimicking cystic neoplasms of the pancreas: CT and MR imaging findings in seven patients. AJR Am J Roentgenol. 2003; 180:195–199.
13. Kim SY, Kim JN, Kwon SO, Cha IH, Ryu SH, Kim YS, Moon JS. A case of duodenal diverticulum mimicking a peripancreatic abscess. Korean J Med. 2013; 84:249–253.
14. Nonaka T, Inamori M, Kessoku T, Ogawa Y, Imajyo K, Yanagisawa S, Shiba T, Sakaguchi T, Nakajima A, Maeda S, et al. Acute obstructive cholangitis caused by an enterolith in a duodenal diverticulum. Endoscopy. 2010; 42:E204–E205.
15. Yoneyama F, Miyata K, Ohta H, Takeuchi E, Yamada T, Kobayashi Y. Excision of a juxtapapillary duodenal diverticulum causing biliary obstruction: report of three cases. J Hepatobiliary Pancreat Surg. 2004; 11:69–72.
16. Chiang TH, Lee YC, Chiu HM, Huang SP, Lin JT, Wang HP. Endoscopic therapeutics for patients with cholangitis caused by the juxtapapillary duodenal diverticulum. Hepatogastroenterology. 2006; 53:501–505.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download