1. Haaf P, Balmelli C, Reichlin T, Twerenbold R, Reiter M, Meissner J, Schaub N, Stelzig C, Freese M, Paniz P, et al. N-terminal pro B-type natriuretic peptide in the early evaluation of suspected acute myocardial infarction. Am J Med. 2011; 124:731–739.
2. Lee DH, Jeong MH, Rhee JA, Choi JS, Lee KH, Lee MG, Sim DS, Park KH, Yoon NS, Yoon HJ, et al. Predictors of long-term survival in acute coronary syndrome patients with left ventricular dysfunction after percutaneous coronary intervention. Korean Circ J. 2012; 42:692–697.
3. Barron HV, Cannon CP, Murphy SA, Braunwald E, Gibson CM. Association between white blood cell count, epicardial blood flow, myocardial perfusion, and clinical outcomes in the setting of acute myocardial infarction: a thrombolysis in myocardial infarction 10 substudy. Circulation. 2000; 102:2329–2334.
4. Han YC, Yang TH, Kim DI, Jin HY, Chung SR, Seo JS, Jang JS, Kim DK, Kim DK, Kim KH, et al. Neutrophil to lymphocyte ratio predicts long-term clinical outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Korean Circ J. 2013; 43:93–99.
5. Palmerini T, Mehran R, Dangas G, Nikolsky E, Witzenbichler B, Guagliumi G, Dudek D, Genereux P, Caixeta A, Rabbani L, et al. Impact of leukocyte count on mortality and bleeding in patients with myocardial infarction undergoing primary percutaneous coronary interventions: analysis from the Harmonizing Outcome with Revascularization and Stent in Acute Myocardial Infarction trial. Circulation. 2011; 123:2829–2837.
6. Kruk M, Przyłuski J, Kalińczuk L, Pregowski J, Kadziela J, Kaczmarska E, Petryka J, Kepka C, Klopotowski M, Chmielak Z, et al. Hemoglobin, leukocytosis and clinical outcomes of ST-elevation myocardial infarction treated with primary angioplasty: ANIN Myocardial Infarction Registry. Circ J. 2009; 73:323–329.
7. Cho KH, Jeong MH, Ahmed K, Hachinohe D, Choi HS, Chang SY, Kim MC, Hwang SH, Park KH, Lee MG, et al. Value of early risk stratification using hemoglobin level and neutrophil-to-lymphocyte ratio in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2011; 107:849–856.
8. Dündar C, Oduncu V, Erkol A, Tanalp AC, Sırma D, Karagöz A, Karabay CY, Kılıçgedik A, Pala S, Tigen K, et al. In-hospital prognostic value of hemoglobin levels on admission in patients with acute ST segment elevation myocardial infarction undergoing primary angioplasty. Clin Res Cardiol. 2012; 101:37–44.
9. Smit JJ, Ottervanger JP, Slingerland RJ, Kolkman JJ, Suryapranata H, Hoorntje JC, Dambrink JH, Gosselink AT, de Boer MJ, Zijlstra F, et al. Comparison of usefulness of C-reactive protein versus white blood cell count to predict outcome after primary percutaneous coronary intervention for ST elevation myocardial infarction. Am J Cardiol. 2008; 101:446–451.
10. Pellizzon GG, Dixon SR, Stone GW, Cox DA, Mattos L, Boura JA, Grines LL, Addala S, O'Neill WW, Grines CL. Relation of admission white blood cell count to long-term outcomes after primary coronary angioplasty for acute myocardial infarction (the Stent PAMI Trial). Am J Cardiol. 2003; 91:729–731.
11. Vis MM, Engström AE, Sjauw KD, Tjong FV, Baan J Jr, Koch KT, DeVries JH, Tijssen JG, de Winter RJ, Piek JJ, et al. Plasma glucose and not hemoglobin or renal function predicts mortality in patients with STEMI complicated with cardiogenic shock. J Cardiovasc Med (Hagerstown). 2010; 11:827–831.
12. Kramer MC, Rittersma SZ, de Winter RJ, Ladich ER, Fowler DR, Liang YH, Kutys R, Carter-Monroe N, Kolodgie FD, van der Wal AC, et al. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J Am Coll Cardiol. 2010; 55:122–132.
13. Heestermans AA, van Werkum JW, Zwart B, van der Heyden JA, Kelder JC, Breet NJ, van't Hof AW, Dambrink JH, Koolen JJ, Brueren BR, et al. Acute and subacute stent thrombosis after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: incidence, predictors and clinical outcome. J Thromb Haemost. 2010; 8:2385–2393.
14. Vagdatli E, Gounari E, Lazaridou E, Katsibourlia E, Tsikopoulou F, Labrianou I. Platelet distribution width: a simple, practical and specific marker of activation of coagulation. Hippokratia. 2010; 14:28–32.
15. Ihara A, Matsumoto K, Kawamoto T, Shouno S, Kawamoto J, Katayama A, Yoshitatsu M, Izutani H. Relationship between hemostatic markers and platelet indices in patients with aortic aneurysm. Pathophysiol Haemost Thromb. 2006; 35:451–456.
16. Ihara A, Kawamoto T, Matsumoto K, Shouno S, Hirahara C, Morimoto T, Noma Y. Relationship between platelet indexes and coronary angiographic findings in patients with ischemic heart disease. Pathophysiol Haemost Thromb. 2006; 35:376–379.
17. Khandekar MM, Khurana AS, Deshmukh SD, Kakrani AL, Katdare AD, Inamdar AK. Platelet volume indices in patients with coronary artery disease and acute myocardial infarction: an Indian scenario. J Clin Pathol. 2006; 59:146–149.
18. Lee JH, Park HS, Chae SC, Cho Y, Yang DH, Jeong MH, Kim YJ, Kim KS, Hur SH, Seong IW, et al. Predictors of six-month major adverse cardiac events in 30-day survivors after acute myocardial infarction (from the Korea Acute Myocardial Infarction Registry). Am J Cardiol. 2009; 104:182–189.
19. Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J. 2000; 21:1502–1513.
20. Sabatine MS, Morrow DA, de Lemos JA, Gibson CM, Murphy SA, Rifai N, McCabe C, Antman EM, Cannon CP, Braunwald E. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002; 105:1760–1763.
21. Thiagarajan RR, Winn RK, Harlan JM. The role of leukocyte and endothelial adhesion molecules in ischemia-reperfusion injury. Thromb Haemost. 1997; 78:310–314.
22. Goel MS, Diamond SL. Neutrophil enhancement of fibrin deposition under flow through platelet-dependent and -independent mechanisms. Arterioscler Thromb Vasc Biol. 2001; 21:2093–2098.
23. Engler RL, Schmid-Schönbein GW, Pavelec RS. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am J Pathol. 1983; 111:98–111.
24. Mann DL, Young JB. Basic mechanisms in congestive heart failure. Recognizing the role of proinflammatory cytokines. Chest. 1994; 105:897–904.
25. Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992; 257:387–389.
26. Levy PS, Kim SJ, Eckel PK, Chavez R, Ismail EF, Gould SA, Ramez Salem M, Crystal GJ. Limit to cardiac compensation during acute isovolemic hemodilution: influence of coronary stenosis. Am J Physiol. 1993; 265:H340–H349.
27. Wahr JA. Myocardial ischaemia in anaemic patients. Br J Anaesth. 1998; 81:10–15.
28. Bae MH, Lee JH, Lee SH, Park SH, Yang DH, Park HS, Cho Y, Jun JE, Chae SC. Serum uric acid as an independent and incremental prognostic marker in addition to N-terminal pro-B-type natriuretic peptide in patients with acute myocardial infarction. Circ J. 2011; 75:1440–1447.
29. Jagroop IA, Clatworthy I, Lewin J, Mikhailidis DP. Shape change in human platelets: measurement with a channelyzer and visualisation by electron microscopy. Platelets. 2000; 11:28–32.
30. Gurm HS, Bhatt DL, Lincoff AM, Tcheng JE, Kereiakes DJ, Kleiman NS, Jia G, Topol EJ. Impact of preprocedural white blood cell count on long term mortality after percutaneous coronary intervention: insights from the EPIC, EPILOG, and EPISTENT trials. Heart. 2003; 89:1200–1204.

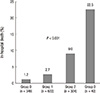









 PDF
PDF ePub
ePub Citation
Citation Print
Print




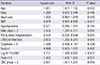
 XML Download
XML Download