Abstract
Papillary thyroid microcarcinoma (PTMC) has been increasing, without a consensus for the management of this condition. In the present study, we analyzed the clinicopathological features of patients with PTMC to examine the impact of initial therapy and establish appropriate treatment. A total of 2,018 patients with PTMC were enrolled at a single university hospital. Of them, 1,245 patients (61.8%) underwent total thyroidectomy, and 1,838 patients (91.3%) underwent central lymph node (LN) dissection. Five-and 10-yr recurrence rates were 3.2% and 4.6%, respectively. In univariate analysis, the prognostic factors for recurrence were N stage, the number of LN metastases, and extrathyroidal extension. However, multivariate analysis revealed LN metastases and N stage as the only significant prognostic factors after adjusting for confounding factors (P < 0.001). Additionally, multivariate analysis of a subgroup consisting of PTMC patients without N1b revealed the number of central LN metastases as the only significant factor. Therefore, intraoperative examination for central LN metastasis may discriminate high or low risk group.
Papillary thyroid cancer (PTC) is the most common endocrine malignancy (1) and accounts for about 80% of thyroid cancers (2). Additionally, papillary thyroid microcarinoma (PTMC) accounts for 36%-60% of thyroid cancers (3, 4). While PTMC is indolent and grows slowly, PTMC with particular biological characteristics, such as clinically apparent node metastasis and extrathyroidal extension, are progressive.
According to several guidelines, total thyroidectomy or completion thyroidectomy is recommended for patients who have PTMC with radiologically or clinically detected cervical nodal metastasis, multifocal malignancy lesions, or extrathyroidal extension (5, 6). However, the management of PTMC varies from observation to total thyroidectomy (3, 7). A lobectomy is preferred over a total thyroidectomy and radioactive iodine (RAI) therapy in Japan, which is not the case in western countries. In addition, central compartment lymph node (LN) management is another subject of debate. The 2009 guideline from the American Thyroid Association (ATA) recommends therapeutic central compartment LN dissection. However, the European Thyroid Association (ETA) recommends prophylactic central compartment LN dissection as a useful strategy for subsequent treatment and follow-up (5, 8). This difference in management may be attributed to varying clinicopathological features among countries. For example, the frequency of the BRAF mutation is 45%-80% and the frequency of LN metastases is 14%-64% (9-12). Therefore, we retrospectively investigated clinicopathological features and prognostic factors for recurrence to determine proper initial surgical therapy in patients with PTMC.
The records were reviewed for all patients with PTMC who were treated at Ajou University Hospital during 1994-2010. There were 2,018 patients who underwent primary surgical therapy at Ajou University Hospital with 5,865 person-years of follow-up. The median follow-up was 3.0 yr, and the longest follow-up was 16 yr. Death information for patients was confirmed from National Cancer Information Center. Death was due to PTC in seven cases.
Comparisons of risk characteristics were performed using the chi-square tests or Fisher's exact test as necessary. Survival from the date of initial surgery to recurrence was analyzed using the Kaplan-Meier method. The log-rank test was used to determine group differences in cumulative recurrence-free curves. All tests were two-sided, with an alpha level of 0.05. All calculations were performed using SPSS 17.0 software (SPSS, Inc. Chicago, IL, USA).
Total thyroidectomy was performed in 61.8% of 2,018 patients with PTMC, and central LN dissection in 1,838 (91.3%) patients. The remaning patients (9.7%) with occult PTMC did not undergo central LN dissection, because of preoperative benign diagnoses of Graves' disease, multinodular goiter, etc. Central LN metastases were present in 593 (29.4%) patients. RAI therapy was performed in 21.9%. Extrathyroidal extension and multiplicity were found in 48.8% and 30.1% respectively. Bilateral cancer of the thyroid was seen in 30.2% of total thyroidectomy. Recurrence from the remnant thyroid accounted for 39% of 41 total recurrences, and a recurrent tumor in a previously dissected central compartment was found in two patients. Lateral cervical LN metastases accounted for 60.9% of recurrences. There were 64 patients for whom the number of central LN metastases was not described in pathological reports. Metachronous distant metastases were found in 7.3% (Table 1).
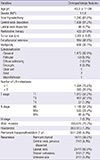
In univariate analysis for recurrence-free rate, extrathyroidal extension, N stage (Fig. 1A) and number of LN metastases (Fig. 1B) were found to be significant prognostic factors; however, neither the extent of thyroidectomy or RAI therapy were significant prognostic factors (Table 2). In additional univariate analysis of a subgroup consisting of PTMC patients with total thyroidectomy and central LN dissection, RAI therapy was not a significant prognostic factor (P = 0.315). In multivariate analysis, both N stage and number of LN metastases were prognostic factors (Table 3). There was no significant difference between the patients without LN metastasis and with one LN metastasis (data not shown). The number of metastatic LN was correlated with bilaterality of cancer (Table 4) and recurrence (Table 5). Therefore, either lobectomy or subtotal thyroidectomy can be performed in patients with one LN metastasis or no LN metastasis.
Controversy exists regarding the extent of thyroidectomy and central LN dissection in patients with PTMC. Retrospective studies have reported that recurrence within the thyroid bed decreases in patients who have undergone total thyroidectomy compared with those treated with subtotal thyroidectomy (13, 14). However, other thyroid cancer specialists have reported that unilateral lobectomy is sufficient as an initial therapy for low-risk patients with papillary and follicular thyroid cancer because of low mortality and high complication rates reported with a more extensive thyroidectomy (15-17).
The frequency of bilateral thyroid carcinoma in low-risk patients who undergo total thyroidectomy is 16.7%-38% (17-20). Among the 1,244 patients in our study, 30.3% had contralateral carcinoma. This incidence rate was similar to previously reported rates for thyroid carcinoma in the contralateral lobe. The recurrence in the remnant contralateral lobe was 1.6% of 772 patients who underwent either lobectomy or subtotal thyroidectomy in our study. The incidence of recurrent thyroid cancer occurring in the residual contralateral lobe of the thyroid gland is 4.7%-46% in such patients (19). In our study, the recurrence rate of the remnant thyroid was lower than previously reported rates. This low recurrence rate may be attributed to our lobectomy criteria. At our institution, lobectomy is performed in patients without clinical LN metastasis, tumor multiplicity, macroscopic extrathyroidal extension and familial history. Another cause of low recurrence in our study may be that the high incidence of bilateral thyroid cancer may not always be clinically significant (20). However, total thyroidectomy can be considered in PTMC patients with more than three central LN metastases, because the recurrence rates between total thyroidectomy and less than total thyroidectomy were not statistically different in our study due to one of several selection (or therapeutic) biases (Table 4, 5) The general consensus is that a central LN dissection is indicated when LN are discovered preoperatively on ultrasound (US) or clinical examination (21, 22). However, prophylactic central LN dissection was performed in patients without clinically apparent central LN metastasis in our institution because of some advantages. Firstly, although US has been regarded as a sensitive imaging modality for LN metastasis screening and diagnosis, it has a low sensitivity in evaluation of LN metastasis in central compartment (23) and the accuracy of preoperative US for central LN metastasis is lower than that of lateral cervical LN metastasis (24). Secondly, central LN dissection was reported to reduce RAI dosage (18) and rate of postoperative detectable thyroglobulin (Tg), which is related with recurrence (25). Thirdly, reoperative central LN dissections represent significant risk of recurrent laryngeal nerve injury and permanent hypoparathyroidism due to the presence of scar tissue and distorted anatomy (26). Fourthly, number of LN metastases was correlated with both recurrence and bilaterality of cancer. Despite these merits of central LN dissection, it is related with the risk of hypoparathyroidism. The frequency of permanent hypoparathyroidism ranges from 2.7%-25% after total thyroidectomy and central compartment LN dissection (18, 27, 28). The frequency of permanent hypoparathyroidism was 4.5% at our institution, which was similar to previous reported rates. If central LN dissections were performed by skilled surgeons, the frequency of permanent hypoparathyroidism may decrease. Therefore, prophylactic central LN dissection can be recommended in PTMC patients. Otherwise, intraoperative examination of ipsilateral central LN metastasis may be useful for prognosis of patients with PTMC and in tailoring postoperative management (29).
This study has several limitations. Firstly, the mean follow-up period was relatively short, because of most of the subjects (88%) had been enrolled after 2006. Secondly, we could not investigate the relationship between LN micrometastasis and recurrence-free rate because usually one or two tissue block from each lymph node had been sliced and stained with hematoxyline-eosin. Thirdly, we could not define the effectiveness of radioactive iodine therapy in intermediate-risk group for recurrence. Therefore, these problems should be investigated in a future prospective randomized controlled study.
In conclusion, lobectomy may be sufficient for initial surgery in selected patients with PTMC, and intraoperative examination for central LN metastasis may be performed to predict the recurrence and to tailor postoperative management.
Figures and Tables
Fig. 1
Recurrence-free rate in patients with papillary thyroid microcarcinoma. (A) Related to N stage, (B) number of LN metastases.

References
1. Vasileiadis I, Karakostas E, Charitoudis G, Stavrianaki A, Kapetanakis S, Kouraklis G, Karatzas T. Papillary thyroid microcarcinoma: clinicopathological characteristics and implications for treatment in 276 patients. Eur J Clin Invest. 2012; 42:657–664.
2. Lee NS, Bae JS, Jeong SR, Jung CK, Lim DJ, Park WC, Kim JS, Kim SN. Risk factors of lymph node metastasis in papillary thyroid microcarcinoma. J Korean Surg Soc. 2010; 78:82–86.
3. Roti E, Rossi R, Trasforini G, Bertelli F, Ambrosio MR, Busutti L, Pearce EN, Braverman LE, Degli Uberti EC. Clinical and histological characteristics of papillary thyroid microcarcinoma: results of a retrospective study in 243 patients. J Clin Endocrinol Metab. 2006; 91:2171–2178.
4. Cho BY. Clinical thyroidology. 3rd ed. Seoul: Korea Medical Book;2010.
5. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.
6. Tuttle RM, Ball DW, Byrd D, Dilawari RA, Doherty GM, Duh QY, Ehya H, Farrar WB, Haddad RI, Kandeel FK, et al. NCCN clinical practice guidelines in oncology: thyroid carcinoma. accessed on 28 November 2013. Available at http://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf.
7. Hay ID, Grant CS, Bergstralh EJ, Thompson GB, van Heerden JA, Goellner JR. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery. 1998; 124:958–964.
8. Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W. European Thyroid Cancer Taskforce. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006; 154:787–803.
9. Zhao Q, Ming J, Liu C, Shi L, Xu X, Nie X, Huang T. Multifocality and total tumor diameter predict central neck lymph node metastases in papillary thyroid microcarcinoma. Ann Surg Oncol. 2013; 20:746–752.
10. Rodriguez JM, Moreno A, Parrilla P, Sola J, Soria T, Tebar FJ, Aranda F. Papillary thyroid microcarcinoma: clinical study and prognosis. Eur J Surg. 1997; 163:255–259.
11. Kim WJ, Bae MJ, Yi YS, Jeon YK, Kim SS, Kim BH, Kim IJ. Clinicopathologic characteristics of papillary microcarcinoma in the elderly. J Korean Thyroid Assoc. 2013; 6:69–74.
12. Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, Ito K, Takami H, Takanashi Y. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003; 237:399–407.
13. Hay ID. Papillary thyroid carcinoma. Endocrinol Metab Clin North Am. 1990; 19:545–576.
14. DeGroot LJ, Kaplan EL, McCormick M, Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990; 71:414–424.
15. Buffet C, Golmard JL, Hoang C, Trésallet C, Du Pasquier Fédiaevsky L, Fierrard H, Aurengo A, Menegaux F, Leenhardt L. Scoring system for predicting recurrences in patients with papillary thyroid microcarcinoma. Eur J Endocrinol. 2012; 167:267–275.
16. Shaha AR. Implications of prognostic factors and risk groups in the management of differentiated thyroid cancer. Laryngoscope. 2004; 114:393–402.
17. Koo BS, Lim HS, Lim YC, Yoon YH, Kim YM, Park YH, Rha KS. Occult contralateral carcinoma in patients with unilateral papillary thyroid microcarcinoma. Ann Surg Oncol. 2010; 17:1101–1105.
18. Bonnet S, Hartl D, Leboulleux S, Baudin E, Lumbroso JD, Al Ghuzlan A, Chami L, Schlumberger M, Travagli JP. Prophylactic lymph node dissection for papillary thyroid cancer less than 2 cm: implications for radioiodine treatment. J Clin Endocrinol Metab. 2009; 94:1162–1167.
19. Rose RG, Kelsey MP, Russell WO, Ibanez ML, White EC, Clark RL. Follow-up study of thyroid cancer treated by unilateral lobectomy. Am J Surg. 1963; 106:494–500.
20. Pasieka JL, Thompson NW, McLeod MK, Burney RE, Macha M. The incidence of bilateral well-differentiated thyroid cancer found at completion thyroidectomy. World J Surg. 1992; 16:711–716.
21. Borson-Chazot F, Causeret S, Lifante JC, Augros M, Berger N, Peix JL. Predictive factors for recurrence from a series of 74 children and adolescents with differentiated thyroid cancer. World J Surg. 2004; 28:1088–1092.
22. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, Mclver B, Sherman SI, Tuttle RM. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006; 16:109–142.
23. Choi JS, Kim J, Kwak JY, Kim MJ, Chang HS, Kim EK. Preoperative staging of papillary thyroid carcinoma: comparison of ultrasound imaging and CT. AJR Am J Roentgenol. 2009; 193:871–878.
24. Shimamoto K, Satake H, Sawaki A, Ishigaki T, Funahashi H, Imai T. Preoperative staging of thyroid papillary carcinoma with ultrasonography. Eur J Radiol. 1998; 29:4–10.
25. Sywak M, Cornford L, Roach P, Stalberg P, Sidhu S, Delbridge L. Routine ipsilateral level VI lymphadenectomy reduces postoperative thyroglobulin levels in papillary thyroid cancer. Surgery. 2006; 140:1000–1005.
26. Kim MK, Mandel SH, Baloch Z, Livolsi VA, Langer JE, Didonato L, Fish S, Weber RS. Morbidity following central compartment reoperation for recurrent or persistent thyroid cancer. Arch Otolaryngol Head Neck Surg. 2004; 130:1214–1216.
27. Pattou F, Combemale F, Fabre S, Carnaille B, Decoulx M, Wemeau JL, Racadot A, Proye C. Hypocalcemia following thyroid surgery: incidence and prediction of outcome. World J Surg. 1998; 22:718–724.
28. Goretzki PE, Simon D, Frilling A, Witte J, Reiners C, Grussendorf M, Horster FA, Röher HD. Surgical reintervention for differentiated thyroid cancer. Br J Surg. 1993; 80:1009–1012.
29. Caliskan M, Park JH, Jeong JS, Lee CR, Park SK, Kang SW, Jeong JJ, Chung WY, Park CS. Role of prophylactic ipsilateral central compartment lymph node dissection in papillary thyroid microcarcinoma. Endocr J. 2012; 59:305–311.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download