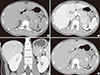
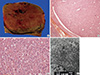
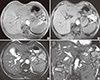
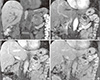
1. Nguyen GK, Vriend R, Ronaghan D, Lakey WH. Heterotopic adrenocortical oncocytoma: a case report with light and electron microscopic studies. Cancer. 1992; 70:2681–2684.
2. Kakimoto S, Yushita Y, Sanefuji T, Kondo A, Fujishima N, Kishikawa M, Matsumoto K. Non-hormonal adrenocortical adenoma with oncocytoma-like appearances. Hinyokika Kiyo. 1986; 32:757–763.
3. Geramizadeh B, Norouzzadeh B, Bolandparvaz S, Sefidbakht S. Functioning adrenocortical oncocytoma: a case report and review of literature. Indian J Pathol Microbiol. 2008; 51:237–239.
4. Akatsu T, Kameyama K, Araki K, Ashizawa T, Wakabayashi G, Kitajima M. Functioning adrenocortical oncocytoma: the first documented case producing interleukin-6 and review of the literature. J Endocrinol Invest. 2008; 31:68–73.
5. Gołkowski F, Buziak-Bereza M, Huszno B, Bałdys-Waligórska A, Stefańska A, Budzyński A, Okoń K, Chrzan R, Urbanik A. The unique case of adrenocortical malignant and functioning oncocytic tumour. Exp Clin Endocrinol Diabetes. 2007; 115:401–404.
6. Xiao GQ, Pertsemlidis DS, Unger PD. Functioning adrenocortical oncocytoma: a case report and review of the literature. Ann Diagn Pathol. 2005; 9:295–297.
7. Erlandson RA, Reuter VE. Oncocytic adrenal cortical adenoma. Ultrastruct Pathol. 1991; 15:539–547.
8. Wong DD, Spagnolo DV, Bisceglia M, Havlat M, McCallum D, Platten MA. Oncocytic adrenocortical neopla: a clinicopathologic study of 13 new cases emphasizing the importance of their recognition. Hum Pathol. 2011; 42:489–499.
9. Korobkin M, Brodeur FJ, Francis IR, Quint LE, Dunnick NR, Londy F. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998; 170:747–752.
10. Bisceglia M, Ludovico O, Di Mattia A, Ben-Dor D, Sandbank J, Pasquinelli G, Lau SK, Weiss LM. Adrenocortical oncocytic tumors: report of 10 cases and review of the literature. Int J Surg Pathol. 2004; 12:231–243.
11. El-Naggar AK, Evans DB, Mackay B. Oncocytic adrenal cortical carcinoma. Ultrastruct Pathol. 1991; 15:549–556.
12. Kitching PA, Patel V, Harach HR. Adrenocortical oncocytoma. J Clin Pathol. 1999; 52:151–153.
13. Defossez SM, Yoder IC, Papanicolaou N, Rosen BR, McGovern F. Nonspecific magnetic resonance appearance of renal oncocytomas: report of 3 cases and review of the literature. J Urol. 1991; 145:552–554.
14. Kim JI, Cho JY, Moon KC, Lee HJ, Kim SH. Segmental enhancement inversion at biphasic multidetector CT: characteristic finding of small renal oncocytoma. Radiology. 2009; 252:441–448.
15. Shah RK, Oto A, Ozkan OS, Ernst RD, Hernandez JA, Chaudhary HB, Koroglu M. Adrenal oncocytoma: US and CT findings. JBR-BTR. 2004; 87:180–182.
16. Mitchell DG, Crovello M, Matteucci T, Petersen RO, Miettinen MM. Benign adrenocortical masses: diagnosis with chemical shift MR imaging. Radiology. 1992; 185:345–351.
17. McLoughlin RF, Bilbey JH. Tumors of the adrenal gland: findings on CT and MR imaging. AJR Am J Roentgenol. 1994; 163:1413–1418.
18. Gandras EJ, Schwartz LH, Panicek DM, Levi G. Case report: adrenocortical oncocytoma: CT and MRI findings. J Comput Assist Tomogr. 1996; 20:407–409.