Abstract
Figures and Tables
Fig. 1

Fig. 2

Fig. 3

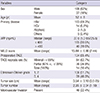
Table 1
Journal List > J Korean Med Sci > v.29(10) > 1022389




Min-Su Park 
https://orcid.org/http://orcid.org/0000-0002-0707-2969
Kwang-Woong Lee 
https://orcid.org/http://orcid.org/0000-0001-6412-1926
Nam-Joon Yi 
https://orcid.org/http://orcid.org/0000-0002-5467-425X
Young Rok Choi 
https://orcid.org/http://orcid.org/0000-0003-2408-7086
Hyeyoung Kim 
https://orcid.org/http://orcid.org/0000-0002-3312-9295
Geun Hong 
https://orcid.org/http://orcid.org/0000-0003-2597-1068
Kyung-Suk Suh 
https://orcid.org/http://orcid.org/0000-0002-9535-7349
Choon-Hyuck David Kwon 
https://orcid.org/http://orcid.org/0000-0002-1082-3321
Jae-Won Joh 
https://orcid.org/http://orcid.org/0000-0003-1732-6210
Suk-Koo Lee 
https://orcid.org/http://orcid.org/0000-0002-8503-2882