Abstract
The University of California, San Francisco, announced in 2011 Cancer of the Prostate Risk Assessment Postsurgical (CAPRA-S) score which included pathologic data, but there were no results for comparing preoperative predictors with the CAPRA-S score. We evaluated the validation of the CAPRA-S score in our institution and compare the result with the preoperative progression predictor, CAPRA score. Data of 130 patients were reviewed who underwent radical prostatectomy for localized prostate cancer from 2008 to 2013. Performance of CAPRA-S score in predicting progression free probabilities was assessed through Kaplan Meier analysis and Cox proportional hazards regression test. Additionally, prediction probability was compared with preoperative CAPRA score by logistic regression analysis. Comparing CAPRA score, the CAPRA-S score showed improved prediction ability for 5 yr progression free survival (concordance index 0.80, P = 0.04). After risk group stratification, 3 group model of CAPRA-S was superior than 3 group model of CAPRA for 3-yr progression free survival and 5-yr progression free survival (concordance index 0.74 vs. 0.70, 0.77 vs. 0.71, P < 0.001). Finally the CAPRA-S score was the more ideal predictor concerned with adjuvant therapy than the CAPRA score through decision curve analysis. The CPARA-S score is a useful predictor for disease progression after radical prostatectomy.
A radical prostatectomy is the most common primary treatment for clinically localized prostate cancer (1). In terms of cancer control, a radical prostatectomy gives good results when the cancer is confined within the prostate (2). Nevertheless, approximately one third of patients will experience biochemical recurrence as shown by prostate-specific antigen (PSA) elevation within 10 yr after a prostatectomy (3, 4, 5, 6). An unfavorable pathology such as extraprostatic disease is detected at prostatectomy in 52% of the patients and is associated with biochemical recurrence (7, 8, 9, 10). Consequently, proper risk stratification after prostatectomy is important for individualized treatment and patient counseling.
To facilitate risk stratification, many nomograms and predictors have been developed. The Cancer of the Prostate Risk Assessment (CAPRA) score is one of them. After the CAPRA score was introduced for preoperative prostate cancer risk stratification, its validity was assessed and relatively good predictability was found (11, 12, 13, 14, 15, 16, 17, 18, 19). To improve the accuracy of prediction, the pathology findings were added which was named as CAPRA-S score was developed by The University of California, San Francisco (13). Recently, its external validity was studied using the Shared Equal Access Regional Cancer Hospital (SEARCH) database and the results validated its effectiveness and ability to predict biochemical recurrence following surgery (20). However, no study has compared the validity of the CAPRA-S score with preoperative predictors in Asian populations.
A total of 130 patients who underwent prostatectomy between 2008 and 2013 by a single surgeon as analyzed retrospectively. The sum of scores of each variables were calculated for CAPRA-S score and CAPRA score. The CAPRA-S scores were calculated using the variables and each scores described in Table 1 (13) and the CAPRA scores were calculated in the same manner with only preoperative variables (21). Fifteen patients were excluded from the CAPRA score group because there were no information about percent positive biopsies. The patients were divided into two groups: each score group and a three-risk level group model (low, intermediate, and high risk). The each score group model means which was stratifying by CAPRA score sum from 0 to 10 point. And of the three-risk group model, low risk groups were 0-2 point and intermediate risk groups were 3-5 point, high risk groups were above 6 point of CAPRA-S score sum (Table 2). Biochemical recurrence after radical prostatectomy was defined as two consecutive PSA values≥0.2 ng/mL at any time postoperatively or any additional treatment more than 6 months after the prostatectomy.
The ability of the CAPRA-S score to predict the 3- and 5-yr progression-free probabilities at our institution was examined. In addition, the 5-yr progression-free probabilities of each score group and the three risk groups were analyzed using Kaplan-Meier analysis and the Cox proportional hazards regression. Finally, the prediction probability of the CAPRA-S score and preoperative CAPRA score were compared through logistic regression analysis with calculated concordance index (c-index) and decision curve analysis. The statistical analysis was supported by Clinical Trail Center, Inje University Busan Paik Hospital.
Recurrence occurred in 13.8% of the 130 patients at a median of 13 months (SD 12.1). There was wide distribution of CAPRA-S scores and 25.4% of the patients had scores>6; these constituted the high-risk group (Table 2).
The 5-yr progression-free probabilities for each CAPRA-S score group and the three risk groups are shown in Table 3 and illustrated in Fig. 1 with Kaplan-Meier curves. For each CAPRA-S score, the progression of disease increased with the risk, although this trend was not significant and had no consistency. Nevertheless, the results from the three-risk-group model showed statistical significance in terms of disease progression with increasing risk. Especially in the high-risk group (CAPRA-S score>6), the 5-yr progression-free probability was 56.9% and its hazard ratio was 13.1 (P=0.014; Fig. 1B).
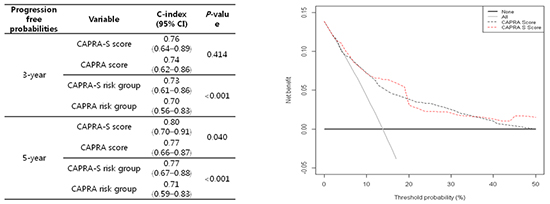
The CAPRA-S score had a higher c-index than the CAPRA score. Except for the 3-yr progression-free probability, the prediction ability of the CAPRA-S score was superior (Table 4). The c-index of each CAPRA-S score group for the 5-yr progression-free probabilities was 0.80, while that of each CAPRA score group was 0.77 (P=0.041). In the three-risk-group model of the CAPRA-S score, the 3- and 5-yr progression-free probabilities were significantly higher than for the CAPRA score. The c-index for the 3- and 5-yr progression-free probabilities of the CAPRA-S score was 0.74 and 0.77, respectively, while that of the CAPRA score was 0.70 and 0.71 (P<0.001).
Finally, the decision curve analysis comparing the CAPRA-S and CAPRA scores indicated a better net benefit of appropriately identifying patients for adjuvant therapy with both scoring systems (Fig. 2). Although the CAPRA-S score did not cover all of the threshold probabilities, it resulted in a more ideal curve than the CAPRA score and both curves did not locate below of the reference curves.
The CAPRA score has been validated externally in the US and European multi-institutional studies with high accuracy with c-indexes ranging from 0.66 to 0.81 (12, 16, 17, 18, 19). Another study demonstrated that it predicted recurrence with the PSA doubling time, metastasis, and cancer-specific mortality (22, 23). Its superiority over other nomograms has been demonstrated (17, 24). The pathology results after radical prostatectomy facilitate prediction of biochemical recurrence and disease progression, so the CAPRA-S score should be better than the CAPRA score for deciding which additional treatments might benefit selected patients (13, 25, 26, 27).
The c-index for the CAPRA-S score in a European multi-institutional study was 0.73 and was higher than for the Stephenson nomogram (20). In our study, the c-index of the CAPRA-S score for the 3- and 5-yr progression-free probabilities was 0.76 and 0.80, respectively. The c-index of the CAPRA-S three risk groups was 0.74 and 0.77, respectively. The progression-free probabilities for each CAPRA-S score was relatively higher in this study compared with the SEARCH and Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) data. We postulated that this result was influenced by the low rate of biochemical recurrences in our patients (13.8%) compared with the SEARCH (33%) and CaPSURE (16%) data. In addition, the small number of patients in our study and the unevenly distributed characteristics might have influenced the results. Also the present study was not a prospective study. Therefore, our study was not representative and had several limitations. Nevertheless, the validity of the CAPRA-S score in our study was clear and its usefulness convincing.
Comparing with preoperative nomograms, the CAPRA score was superior (17, 24). We also compared the CAPRA and CAPRA-S scores, which were constructed from similar items and patients, and examined whether the performance of CAPRA-S score was more accurate. For the three risk groups, the CAPRA-S score had significantly (P<0.001) greater predictive ability for the 3- and 5-yr progression-free probabilities (0.74 and 0.77, respectively) than did the CAPRA score (0.70 and 0.71, respectively). In the decision curve analysis, both the CAPRA and CAPRA-S scores had a better net benefit of appropriately identifying patients for adjuvant therapy. The CAPRA-S score traced a more ideal curve than the CAPRA score, as no point below the reference lines was seen. However, neither was clearly superior.
Despite the high accuracy of the CAPRA-S score for predicting the progression of prostate cancer after prostatectomy, Korean results, including our study, gave different survival curves and progression-free probabilities compared to results using SEARCH and CaPSURE data. The first Korean study of the CAPRA-S score found that the survival curves for the progression-free probabilities were inconsistent (28). We also obtained similar results for inconsistence about survival curve of progression-free probabilities using CAPRA-S score. We must consider the baseline characteristics of the patients. In the two Korean studies including present study, high-risk prostate patients comprised 35.8% and 25.4% of the total, compared 17% for the SEARCH data and for 6.5% CaPSURE (13, 20, 28). Although the Korean studies enrolled fewer patients, the race- and nation-specific characteristics of prostate cancer should be considered to develop a more accurate prediction model. Koreans with prostate cancer in whom a radical prostatectomy is indicated had poorer characteristics than Americans (29). It is important that a more specific prediction model in terms of race and nation be developed. Just as no nomograms have shown absolute prediction ability, the CAPRA-S score might also need continuous correction.
Although biochemical recurrence or disease progression does not correlate with the mortality of prostate cancer directly, adjuvant therapy might be required given poor pathology results. As the decision curve analysis showed, the CAPRA-S score should be able to stratify the patients who need adjuvant therapy after radical prostatectomy with good predictability more easily than CAPRA score. A large-scale study should examine the performance of the CAPRA-S score in stratifying patients who need adjuvant therapy.
Conclusively, the CAPRA-S score, which includes pathology results, is a useful predictor of prostate cancer progression after radical prostatectomy compared with the CAPRA score. It may help clinicians and patients to decide on adjuvant therapy after surgery. After further revision according to race or national characteristics, the CAPRA-S score might also be useful in other countries with an increasing incidence of prostate cancer, as in Korea.
Figures and Tables
Fig. 1
The 5-yr biochemical-progression-free probabilities stratified by CAPRA-S score group (A) and the three-risk-group model (B) using Kaplan-Meier curves.

Fig. 2
Decision curve analysis comparing the CAPRA-S and CAPRA scores. The y axis shows the net increase in the proportion of patients appropriately identified for adjuvant treatment. The solid black line represents the strategy involving treating all patients with adjuvant therapy (assuming all will experience recurrence) and the solid gray line represents the strategy involving treating no patients (assuming none will experience recurrence).

References
1. Meltzer D, Egleston B, Abdalla I. Patterns of prostate cancer treatment by clinical stage and age. Am J Public Health. 2001; 91:126–128.
2. National Institutes of Health Consensus Development Panel. Consensus statement: the management of clinically localized prostate cancer. NCI Monogr. 1988; (7):3–6.
3. Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy: patterns of recurrence and cancer control. Urol Clin North Am. 1997; 24:395–406.
4. Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004; 172:910–914.
5. Zincke H, Oesterling JE, Blute ML, Bergstralh EJ, Myers RP, Barrett DM. Long-term (15 years) results after radical prostatectomy for clinically localized (stage T2c or lower) prostate cancer. J Urol. 1994; 152:1850–1857.
6. Catalona WJ, Smith DS. 5-year tumor recurrence rates after anatomical radical retropubic prostatectomy for prostate cancer. J Urol. 1994; 152:1837–1842.
7. Ward JF, Zincke H, Bergstralh EJ, Slezak JM, Myers RP, Blute ML. The impact of surgical approach (nerve bundle preservation versus wide local excision) on surgical margins and biochemical recurrence following radical prostatectomy. J Urol. 2004; 172:1328–1332.
8. Bott SR, Freeman AA, Stenning S, Cohen J, Parkinson MC. Radical prostatectomy: pathology findings in 1001 cases compared with other major series and over time. BJU Int. 2005; 95:34–39.
9. Antonarakis ES, Feng Z, Trock BJ, Humphreys EB, Carducci MA, Partin AW, Walsh PC, Eisenberger MA. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int. 2012; 109:32–39.
10. Bottke D, Golz R, Störkel S, Hinke A, Siegmann A, Hertle L, Miller K, Hinkelbein W, Wiegel T. Phase 3 study of adjuvant radiotherapy versus wait and see in pT3 prostate cancer: impact of pathology review on analysis. Eur Urol. 2013; 64:193–198.
11. Budäus L, Isbarn H, Tennstedt P, Salomon G, Schlomm T, Steuber T, Haese A, Chun F, Fisch M, Michl U, et al. Risk assessment of metastatic recurrence in patients with prostate cancer by using the Cancer of the Prostate Risk Assessment score: results from 2937 European patients. BJU Int. 2012; 110:1714–1720.
12. Cooperberg MR, Freedland SJ, Pasta DJ, Elkin EP, Presti JC Jr, Amling CL, Terris MK, Aronson WJ, Kane CJ, Carroll PR. Multiinstitutional validation of the UCSF cancer of the prostate risk assessment for prediction of recurrence after radical prostatectomy. Cancer. 2006; 107:2384–2391.
13. Cooperberg MR, Hilton JF, Carroll PR. The CAPRA-S score: a straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer. 2011; 117:5039–5046.
14. Ishizaki F, Hoque MA, Nishiyama T, Kawasaki T, Kasahara T, Hara N, Takizawa I, Saito T, Kitamura Y, Akazawa K, et al. External validation of the UCSF-CAPRA (University of California, San Francisco, Cancer of the Prostate Risk Assessment) in Japanese patients receiving radical prostatectomy. Jpn J Clin Oncol. 2011; 41:1259–1264.
15. Loeb S, Carvalhal GF, Kan D, Desai A, Catalona WJ. External validation of the cancer of the prostate risk assessment (CAPRA) score in a single-surgeon radical prostatectomy series. Urol Oncol. 2012; 30:584–589.
16. Seo WI, Kang PM, Chung JI. Predictive value of the cancer of the prostate risk assessment score for recurrence-free survival after radical prostatectomy in Korea: a single-surgeon series. Korean J Urol. 2014; 55:321–326.
17. Lughezzani G, Budäus L, Isbarn H, Sun M, Perrotte P, Haese A, Chun FK, Schlomm T, Steuber T, Heinzer H, et al. Head-to-head comparison of the three most commonly used preoperative models for prediction of biochemical recurrence after radical prostatectomy. Eur Urol. 2010; 57:562–568.
18. May M, Knoll N, Siegsmund M, Fahlenkamp D, Vogler H, Hoschke B, Gralla O. Validity of the CAPRA score to predict biochemical recurrence-free survival after radical prostatectomy: results from a european multicenter survey of 1,296 patients. J Urol. 2007; 178:1957–1962.
19. Zhao KH, Hernandez DJ, Han M, Humphreys EB, Mangold LA, Partin AW. External validation of University of California, San Francisco, Cancer of the Prostate Risk Assessment score. Urology. 2008; 72:396–400.
20. Punnen S, Freedland SJ, Presti JC Jr, Aronson WJ, Terris MK, Kane CJ, Amling CL, Carroll PR, Cooperberg MR. Multi-institutional validation of the CAPRA-S score to predict disease recurrence and mortality after radical prostatectomy. Eur Urol. 2014; 65:1171–1177.
21. Cooperberg MR, Pasta DJ, Elkin EP, Litwin MS, Latini DM, Du Chane J, Carroll PR. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005; 173:1938–1942.
22. Schroeck FR, Aronson WJ, Presti JC Jr, Terris MK, Kane CJ, Amling CL, Freedland SJ. Do nomograms predict aggressive recurrence after radical prostatectomy more accurately than biochemical recurrence alone? BJU Int. 2009; 103:603–608.
23. Cooperberg MR, Broering JM, Carroll PR. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J Natl Cancer Inst. 2009; 101:878–887.
24. Vickers A. Prediction models in urology: are they any good, and how would we know anyway? Eur Urol. 2010; 57:571–573.
25. Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, di'Sant-Agnese PA, Trump D. Eastern Cooperative Oncology Group study EST 3886. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006; 7:472–479.
26. Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, Messing E, Forman J, Chin J, Swanson G, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009; 181:956–962.
27. Stephenson AJ, Scardino PT, Kattan MW, Pisansky TM, Slawin KM, Klein EA, Anscher MS, Michalski JM, Sandler HM, Lin DW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007; 25:2035–2041.
28. Seong KT, Lim JH, Park CM, Kim HK, Park JY. External validation of the cancer of the prostate risk assessment-S score in Koreans undergoing radical prostatectomy. Korean J Urol. 2013; 54:433–436.
29. Kang DI, Chung JI, Ha HK, Min K, Yoon J, Kim W, Seo WI, Kang PM, Jung SJ, Kim IY. Korean prostate cancer patients have worse disease characteristics than their American counterparts. Asian Pac J Cancer Prev. 2013; 14:6913–6917.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download