Abstract
Previous studies suggest that maternal characteristics may be associated with neonatal outcomes. However, the influence of maternal characteristics on birth weight (BW) has not been adequately determined in Korean populations. We investigated associations between maternal characteristics and BW in a sample of 813 Korean women living in the Seoul metropolitan area, Korea recruited using data from the prospective hospital-based COhort for Childhood Origin of Asthma and allergic diseases (COCOA) between 2007 and 2011. The mean maternal age at delivery was 32.3 ± 3.5 yr and prepregnancy maternal body mass index (BMI) was 20.7 ± 2.5 kg/m2. The mean BW of infant was 3,196 ± 406 g. The overall prevalence of a maternal history of allergic disease was 32.9% and the overall prevalence of allergic symptoms was 65.1%. In multivariate regression models, prepregnancy maternal BMI and gestational age at delivery were positively and a maternal history of allergic disease and nulliparity were negatively associated with BW (all P < 0.05). Presence of allergic symptoms in the mother was not associated with BW. In conclusion, prepregnancy maternal BMI, gestational age at delivery, a maternal history of allergic disease, and nulliparity may be associated with BW, respectively.
Birth weight (BW) has long been recognized as a good surrogate marker for fetal well-being (1) as well as a key determinant of neonatal mortality, morbidity, subsequent growth and development, and an early onset of adulthood disease (2). Low birth weight (LBW) is associated with inadequate fetal nutritional status and growth restriction, and increases the risk of serious neonatal morbidity or death (1), whereas macrosomia is associated with an increased risk of obstetric complications, such as birth injury and cesarean section (3). BW is also a predictor of allergic diseases, obesity, diabetes mellitus, and cardiovascular disease in later life (4-7).
Previous studies have demonstrated direct and indirect influences of genetic-, socio-cultural-, demographic-, and behavioral maternal factors on BW (8-10). For example, advanced maternal age (11), parity (12), and prepregnancy body mass index (BMI) (13) have a substantial impact on BW. Maternal education, household income, and smoking and drinking habits also impact on BW (8). However, these associations have not been adequately determined in Korean populations. Such research is important, since modern Korean society is rapidly adopting Western patterns of food consumption and lifestyle, which has a substantial influence on weight.
The aim of the present study was to determine the effects of specific maternal characteristics on BW in a Korean population. The null hypothesis was that maternal characteristics are not correlated with BW in the Korean population.
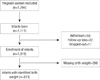
The COhort for Childhood Origin of Asthma and allergic diseases (COCOA) is an ongoing prospective hospital-based birth cohort study. Its aim is to examine the relationship between maternal lifestyle and the subsequent development of allergic disease in the child in the Korean population. The present study investigated 1,294 unselected pregnant women from this cohort, who attended four different hospitals and seven Public Health Centers for antenatal care located in the Seoul metropolitan area, Korea between November 2007 and September 2011. Subjects with diabetes, preeclampsia, anemia, severe infections, etc. during pregnancy that might have an effect on the development of allergic disease were excluded from recruiting. Each subject completed a modified version of the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire (14) at 36 weeks gestation. The questionnaire items concerned the demographic data and medical histories of the pregnant women, including any history of allergic disease, and environmental factors and dietary patterns. The newborns were measured stretched in supine position using an infant anthropometer by trained nurses in the delivery room, as described previously (15). Details of the subsequent delivery were recorded at birth. During the study period, 1,111 neonates were born. Among them, fifty nine subjects withdrew their consent, 22 were lost to follow-up, and 11 dropped out, yielding 1,019 neonates (Fig. 1). The statistical analyses of the present study included the data of all the mother and infant for whom both a completed ISAAC questionnaire and delivery records such as birth weight were available (n = 813; 62.8%). The mother was asked to report her height (in cm) and asked 'Do you know how much you weighed before you became pregnant?' She could answer 'I do not know' or report her weight (in kg). Prepregnancy BMI was calculated as prepregnancy body weight in kilograms divided by height in meters squared (kg/m2).
This study was approved by the institutional review board of the Asan Medical Center (IRB No. 2008-0616), the Samsung Medical Center (IRB No. 2009-02-021), the Severance Hospital (IRB No. 4-2008-0588) and the CHA Medical Center (IRB No. 2010-010). Informed consent was confirmed by each IRB and obtained from the parents of each infant.
The data were analyzed by SAS package (SAS Institute, Cary, NC, USA) for Windows. Analyses included frequencies and cross-tabulations for categorical data, and means and standard deviation for continuous data. Univariate and multivariate regression analyses were computed in order to test the impact of maternal characteristics on newborn BW. Type of occupation was classified into expert (assemblymen, executives, or administrators), non-expert (repairmen, clerks, service industry workers, retailers, farmers, forestry technicians, fishermen, assembly technicians, unskilled workers, professional soldiers, etc.) and jobless (full-time homemaker). In univariate and multivariate regression analyses, jobless (full-time homemaker) was reclassified as non-expert. Maternal education was classified into high school or less, college graduate, and university graduate or higher.
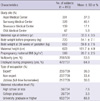
Data on BW were available for 813 neonates (62.8%). Table 1 shows the characteristics of the women in the study sample. The mean maternal weight before pregnancy and at 26 weeks of gestation was 54.1 ± 7.1 kg and 59.8 ± 7.5 kg, respectively. Table 2 shows the prevalence of allergic disease and lifestyle factors of the women in the study. The percentages of subjects with a history of asthma, allergic rhinitis, allergic conjunctivitis, atopic dermatitis, and food allergy were 2.0%, 22.0%, 9.9%, 7.9%, and 5.8%, respectively, thereby yielding the overall prevalence of a history of allergic disease of 32.9%. The percentages of subjects with symptoms of asthma, allergic rhinitis, allergic conjunctivitis, atopic dermatitis, and food allergy during pregnancy were 5.1%, 58.7%, 8.3%, 12.6%, and 4.6%, respectively, thereby yielding the overall prevalence of allergic symptoms of 65.1%. A total of 6.6% of the women had smoked until pregnancy, 0.4% had smoked during pregnancy, and 62.7% had been exposed to passive smoking during pregnancy. The percentage of women who had drunk alcohol during pregnancy was 11.2%. Table 3 shows the neonatal outcomes including gender, gestational age (GA), BW, length, and head circumference at delivery. A total of 48.2% of the infants were male. Mean GA at delivery was 39.2 ± 1.2 weeks, mean BW was 3,196 ± 406 g, mean length was 49.5 ± 1.9 cm, and mean head circumference was 34.3 ± 1.3 cm. The percentages of offspring with a BW of < 2,500 g, 2,500-4,000 g, and ≥ 4,000 g were 5.0%, 92.6%, and 2.4%, respectively.
Univariate linear regression analysis showed that prepregnancy maternal BMI and GA were positively associated with BW (β = 18.29, P = 0.006 for BMI and β = 145.38, P < 0.001 for GA, respectively) and gender (female), a maternal history of allergic disease, and nulliparity were negatively associated with BW (β = -58.28, P = 0.042 for gender [female], β = -72.53, P = 0.022 for a history of allergic disease, and β = -122.07, P = 0.001 for nulliparity, respectively) (Table 4). Multivariate linear regression analysis showed that prepregnancy maternal BMI and GA were positively associated with BW (β = 21.60, P = 0.009 for BMI and β = 170.64, P < 0.001 for GA, respectively) and a maternal history of allergic disease and nulliparity were negatively associated with BW (β = -117.49, P = 0.009 for maternal history of allergic disease and β = -176.05, P < 0.001 for nulliparity, respectively) (Table 4).
The aim of this large prospective hospital-based birth cohort study was to determine the effect of maternal characteristics such as maternal age, prepregnancy BMI, smoking, parity, and allergy on BW in a Korean population living in the Seoul metropolitan area, Korea. In multivariate regression models, prepregnancy maternal BMI and GA were positively associated with BW (β = 21.60, P = 0.009 for BMI and β = 170.64, P < 0.001 for GA, respectively) and a maternal history of allergic disease and nulliparity were negatively associated with BW (β = -117.49, P = 0.009 for a maternal history of allergic disease and β = -176.05, P < 0.001 for nulliparity).
The present results correspond well with those of previous studies, which reported a significant association between prepregnancy BMI and adiposity in the offspring (13). Several underlying mechanisms have been proposed. First, maternal size may determine fetal size via protein-energy availability (16). If a prepregnancy energy deficiency persists during early pregnancy-when energy requirements are at their greatest-the substrates necessary to maintain appropriate fetal tissue growth will be unavailable, thereby resulting in LBW. Second, poor maternal nutritional status during pregnancy is associated with reduced placental weight and surface area (17), which may limit nutrient transfer from the maternal circulation to the fetus, despite increased dietary intake later in pregnancy. Whether a smaller placenta is a direct result of maternal malnutrition or whether the effect is mediated through disruption of endocrine function remains unknown. However, reduced concentrations of hormones such as leptin and estrogen may contribute to fetal growth restriction (18). Third, protein-energy malnutrition may not entirely explain the association between prepregnancy BMI and BW (19). A previous study suggested that underweight women have a lower total plasma volume during early and late pregnancy compared with normal and overweight women, which results in a proportionately reduced cardiac output. This would result in lower uteroplacental blood flow and, hence, a decrease in the transfer of nutrients to the fetus and a reduction in fetal growth (19). Fourth, in underweight women, inadequate micronutrient intake during pregnancy may be associated with reduced maternal plasma volume and BW. Fifth, there is evidence suggesting that intrinsic maternal control of fetal size correlates with maternal size (20).
The prevalence of allergic disease is increasing worldwide (21, 22). However, no previous study has focused on the impact of allergic disease during pregnancy on BW in Korean populations. We found that a maternal history of allergic disease may be negatively associated with BW. Previous studies have found conflicting results concerning the impact of maternal allergic diseases on BW (1, 23-27). Asthma during pregnancy is associated with physiological changes in the expectant mother (28). However, the effect of asthma during pregnancy on BW is poorly characterized. Although some studies found no association between maternal asthma and BW (25), others reported lower BW in infants born to asthmatic women (26). Severe exacerbations of asthma or moderate to severe asthma during pregnancy have been shown to increase the risk of LBW (29), while maternal forced expiratory volume in one second is directly positively associated with BW (23). Chronic hypoxia secondary to uncontrolled asthma and the impact of corticosteroids may explain LBW in infants born to asthmatic mothers (24). The association between atopic dermatitis in pregnancy and BW is complex. Pregnant women may avoid certain foods in the misapprehension that they might cause atopic dermatitis in their offspring, thereby resulting in LBW of their offspring. Severe exacerbations of atopic dermatitis during pregnancy may also increase the risk of LBW. A recent study reported that although the use of potent or super potent topical corticosteroids during pregnancy was not associated with severe neonatal outcomes such as orofacial cleft, preterm delivery, or fetal death, it was associated with a significantly increased risk of LBW (30). There are several possible mechanisms for this. Topical corticosteroids affect the insulin-like growth factor system, and may thus result in fetal growth restriction (31). Corticosteroids also induce dysregulation of placental hormone/cytokine gene expression and downregulation of the insulin-like growth factor-II/Akt signaling pathway, which may result in increased placental apoptosis, placental insufficiency, and fetal growth restriction (32). Although some studies showed that the prevalence of allergic rhinitis was lower in mothers of extremely LBW ( < 1,000 g) pre-term infants compared with mothers of full-term babies (33), others reported no association between maternal allergic rhinitis during pregnancy and BW (27). The association between maternal allergic disease and BW of infant may be explained by the Developmental Origins of Health and Disease (DOHaD) theory (34). The concept of this theory is that human development in utero or during the early phases of extrauterine life affects risk of noncommunicable diseases (NCDs), such as cardiovascular diseases, diabetes, chronic lung disease, allergy, some forms of cancer, cognitive decline, osteoporosis, sarcopenia, and effective disorders in later life (34). According to this theory, an insult such as maternal allergic disease at a critical time of fetal growth and development may have a permanent impact on the body's structure, physiology, and metabolism, thereby resulting in a certain BW, which is considered a predictor of many NCDs (4-7).
An important finding worth mentioning in this study is that a maternal history of allergic disease was associated with BW, whereas the presence of allergic symptoms was not associated with BW. Possible explanations are as follows: 1) Maternal history of allergic disease itself may have a significantly greater impact on the fetal development in utero or it may be the result of prolonged effect imposed on the fetus by maternal allergic disease through immunological or unknown mechanisms. On the contrary, the presence of allergic symptoms refers to the presence of allergic symptoms in the past 12 months, which therefore may have a different effect on the fetus in utero, thereby resulting in disparate perinatal outcomes: 2) Pregnant women with controlled allergic disease may have reported no presence of allergic symptoms: and 3) Also, there is a possibility that a large number of vasomotor rhinitis, intrinsic asthma, infectious conjunctivitis, and seborrheic dermatitis cases may have been misinterpreted to be atopic diseases by the mother. In fact, 65.1% of the women reported to have the presence of allergic symptoms, whereas merely 32.9% of the women reported to have a history of allergic disease.
The present study demonstrated that nulliparity was associated with lower birth weight. This finding is in line with a meta-analysis of 41 studies (12) reporting that nulliparity was associated with increased odds of low birth weight and small-for-gestational-age and reduction in birth weight. This may be due to a reduced uterine capacity and blood supply, and the consequent reduction in the delivery of oxygen and nutrients to the developing fetus (35).
The strengths of the present study are its longitudinal design and the large study population. The observed associations are not explained by confounding factors such as maternal education and types of occupation, since these variables were included in all analyses.
The present study has a few limitations. First, prepregnancy weight was self-reported at recruitment, which occurred at 26 weeks gestation. Women are likely to underreport having been overweight (36). The fact that weight was reported retrospectively may have further contributed to this underestimation of prepregnancy weight. This bias may have attenuated the association between high prepregnancy BMI and BW. A second limitation is that postpartum weight was not measured and consequently weight gain during the entire pregnancy was not assessed.
In conclusion, the present results confirm the hypothesis that prepregnancy maternal BMI and GA are positively and nulliparity is negatively associated with BW, and expand previous findings by showing that a maternal history of allergic disease has a negative effect on BW. Maintaining maternal BMI in the normal range during the preconception period may ensure good neonatal outcomes such as normal BW.
Figures and Tables
Table 2
Prevalence of allergic disease and lifestyle factors in the pregnant women in the present study

ACKNOWLEDGMENTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Aly H, Nada A, Ahmad T, Mohamed M, Massaro AN, Bathgate S, Macri CJ, Larsen JW Jr. Maternal asthma, race and low birth weight deliveries. Early Hum Dev. 2011. 87:457–460.
2. Elizabeth KE, Krishnan V, Zachariah P. Auxologic, biochemical and clinical (ABC) profile of low birth weight babies- a 2-year prospective study. J Trop Pediatr. 2007. 53:374–382.
3. Heslehurst N, Simpson H, Ells LJ, Rankin J, Wilkinson J, Lang R, Brown TJ, Summerbell CD. The impact of maternal BMI status on pregnancy outcomes with immediate short-term obstetric resource implications: a meta-analysis. Obes Rev. 2008. 9:635–683.
4. Dratva J, Breton CV, Hodis HN, Mack WJ, Salam MT, Zemp E, Gilliland F, Kuenzli N, Avol E. Birth weight and carotid artery intima-media thickness. J Pediatr. 2012. doi: 10.1016/j.jpeds.2012.10.060.
5. Lau C, Rogers JM, Desai M, Ross MG. Fetal programming of adult disease: implications for prenatal care. Obstet Gynecol. 2011. 117:978–985.
6. Tedner SG, Örtqvist AK, Almqvist C. Fetal growth and risk of childhood asthma and allergic disease. Clin Exp Allergy. 2012. 42:1430–1447.
7. Lundholm C, Ortqvist AK, Lichtenstein P, Cnattingius S, Almqvist C. Impaired fetal growth decreases the risk of childhood atopic eczema: a Swedish twin study. Clin Exp Allergy. 2010. 40:1044–1053.
8. Emanuel I, Kimpo C, Moceri V. The association of maternal growth and socio-economic measures with infant birthweight in four ethnic groups. Int J Epidemiol. 2004. 33:1236–1242.
9. Astone NM, Misra D, Lynch C. The effect of maternal socio-economic status throughout the lifespan on infant birthweight. Paediatr Perinat Epidemiol. 2007. 21:310–318.
10. Jackson DJ, Batiste E, Rendall-Mkosi K. Effect of smoking and alcohol use during pregnancy on the occurrence of low birthweight in a farming region in South Africa. Paediatr Perinat Epidemiol. 2007. 21:432–440.
11. Ludford I, Scheil W, Tucker G, Grivell R. Pregnancy outcomes for nulliparous women of advanced maternal age in South Australia, 1998-2008. Aust N Z J Obstet Gynaecol. 2012. 52:235–241.
12. Shah PS. Knowledge Synthesis Group on Determinants of LBW/PT births. Parity and low birth weight and preterm birth: a systematic review and meta-analyses. Acta Obstet Gynecol Scand. 2010. 89:862–875.
13. Liu Y, Dai W, Dai X, Li Z. Prepregnancy body mass index and gestational weight gain with the outcome of pregnancy: a 13-year study of 292,568 cases in China. Arch Gynecol Obstet. 2012. 286:905–911.
14. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998. 351:1225–1232.
15. Laron Z. The diagnostic and prognostic importance of neonatal length measurements. Isr Med Assoc J. 2000. 2:84–85.
16. Neggers Y, Goldenberg RL. Some thoughts on body mass index, micronutrient intakes and pregnancy outcome. J Nutr. 2003. 133:1737S–1740S.
17. Lechtig A, Yarbrough C, Delgado H, Martorell R, Klein RE, Béhar M. Effect of moderate maternal malnutrition on the placenta. Am J Obstet Gynecol. 1975. 123:191–201.
18. Mantzoros CS. Role of leptin in reproduction. Ann N Y Acad Sci. 2000. 900:174–183.
19. Rosso P, Salas SP. Nutrient regulation during pregnancy, lactation, and infant growth. 1994. New York: Plenum Press.
20. Sebire NJ, Jolly M, Harris J, Regan L, Robinson S. Is maternal underweight really a risk factor for adverse pregnancy outcome? a population-based study in London. BJOG. 2001. 108:61–66.
21. Hong SJ, Lee MS, Sohn MH, Shim JY, Han YS, Park KS, Ahn YM, Son BK, Lee HB. Korean ISAAC Study Group. Self-reported prevalence and risk factors of asthma among Korean adolescents: 5-year follow-up study, 1995-2000. Clin Exp Allergy. 2004. 34:1556–1562.
22. Yu JS, Lee CJ, Lee HS, Kim J, Han Y, Ahn K, Lee SI. Prevalence of atopic dermatitis in Korea: analysis by using national statistics. J Korean Med Sci. 2012. 27:681–685.
23. Schatz M, Dombrowski MP, Wise R, Momirova V, Landon M, Mabie W, Newman RB, Rouse DJ, Lindheimer M, Miodovnik M, et al. Spirometry is related to perinatal outcomes in pregnant women with asthma. Am J Obstet Gynecol. 2006. 194:120–126.
24. Dombrowski MP. Outcomes of pregnancy in asthmatic women. Immunol Allergy Clin North Am. 2006. 26:81–92.
25. Kelly YJ, Brabin BJ, Milligan P, Heaf DP, Reid J, Pearson MG. Maternal asthma, premature birth, and the risk of respiratory morbidity in school-children in Merseyside. Thorax. 1995. 50:525–530.
26. Firoozi F, Lemière C, Beauchesne MF, Perreault S, Forget A, Blais L. Impact of maternal asthma on perinatal outcomes: a two-stage sampling cohort study. Eur J Epidemiol. 2012. 27:205–214.
27. Somoskövi A, Bártfai Z, Tamási L, Kocsis J, Puhó E, Czeizel AE. Population-based case-control study of allergic rhinitis during pregnancy for birth outcomes. Eur J Obstet Gynecol Reprod Biol. 2007. 131:21–27.
28. Belanger K, Hellenbrand ME, Holford TR, Bracken M. Effect of pregnancy on maternal asthma symptoms and medication use. Obstet Gynecol. 2010. 115:559–567.
29. Namazy JA, Murphy VE, Powell H, Gibson PG, Chambers C, Schatz M. Effects of asthma severity, exacerbations and oral corticosteroids on perinatal outcomes. Eur Respir J. 2012. doi: 10.1183/09031936.00195111.
30. Chi CC, Mayon-White RT, Wojnarowska FT. Safety of topical corticosteroids in pregnancy: a population-based cohort study. J Invest Dermatol. 2011. 131:884–891.
31. Randhawa R, Cohen P. The role of the insulin-like growth factor system in prenatal growth. Mol Genet Metab. 2005. 86:84–90.
32. Ain R, Canham LN, Soares MJ. Dexamethasone-induced intrauterine growth restriction impacts the placental prolactin family, insulin-like growth factor-II and the Akt signaling pathway. J Endocrinol. 2005. 185:253–263.
33. Savilahti E, Siltanen M, Pekkanen J, Kajosaari M. Mothers of very low birth weight infants have less atopy than mothers of full-term infants. Clin Exp Allergy. 2004. 34:1851–1854.
34. Hanson M, Gluckman P. Developmental origins of noncommunicable disease: population and public health implications. Am J Clin Nutr. 2011. 94:1754S–1758S.
35. Zhang X, Mumford SL, Cnattingius S, Schisterman EF, Kramer MS. Reduced birthweight in short or primiparous mothers: physiological or pathological? BJOG. 2010. 117:1248–1254.
36. Scholtens S, Wijga AH, Brunekreef B, Kerkhof M, Postma DS, Oldenwening M, de Jongste JC, Smit HA. Maternal overweight before pregnancy and asthma in offspring followed for 8 years. Int J Obes (Lond). 2010. 34:606–613.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download