Abstract
The identification of mediastinal lymph nodes (LNs) in lung cancer is an important step of treatment decision and prognosis prediction. The endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is widely used to assess the mediastinal LNs and tissue confirmation in lung cancer. As use of bronchoscopy or EBUS-TBNA has been increased, bronchial anthracofibrosis (BAF) has been detected frequently. Moreover, BAF is often accompanied by mediastinal lymphadenopathy and showed false-positive positron emission tomography uptake, which mimics metastatic lymphadenopathy in lung cancer patients. However, clinical implication of BAF during bronchoscopy is not well understood in lung cancer. We retrospectively reviewed 536 lung cancer patients who performed EBUS-TBNA and observed BAF in 55 patients. A total of 790 LNs were analyzed and macroscopic tissue pigmentation was observed in 228 patients. The adjusted odds ratio for predicting malignant LN was 0.46 for BAF, and 0.22 for macroscopic tissue pigmentation. The specificity of BAF and macroscopic tissue pigmentation for predicting a malignant LN was 75.7% and 42.2%, respectively, which was higher than the specificity of using LN size or standard uptake value on PET. In conclusion, BAF and macroscopic tissue pigmentation during EBUS-TBNA are less commonly found in malignant LNs than reactive LNs in Korean lung cancer patients.
Lung cancer has poor prognosis with only 15% of 5-yr survival rate. The treatment of lung cancer is a local removal with surgery or radiotherapy if it is indicated (1). The assessment of mediastinal lymph nodes (LNs) in lung cancer is a critical step of deciding whether surgical resection is appropriate or not. The use of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is on the increase because of an accuracy and minimal invasiveness in accessing mediastinal LNs (2). Before performing EBUS-TBNA, standard bronchoscopy is usually done to identify any endobronchial lesion. In addition, bronchoscopy is generally performed during the evaluation of lung cancer stage based on the National Comprehensive Cancer Network (NCCN) guidelines (1). Bronchial anthracofibrosis (BAF) is a bronchoscopically visible anthracotic pigmentation associated with the narrowing or obliteration of the bronchi. As a bronchoscopy or EBUS-TBNA is widely performed, a detection of BAF becomes easier and more frequent. However, clinical implication of BAF is not completely understood.
BAF is considered to be associated with chronic biomass-fuel smoke exposure as well as active or old tuberculosis (3, 4). Because Korea is an endemic area of tuberculosis, BAF is frequently found during bronchoscopy or EBUS. Although a large scale investigation has not be done, Jang et al. (5) reported the prevalence of anthracofibrosis as 4.2% among the all patients who underwent a bronchoscopy. Mediastinal lymphadenopathy with calcification is a common co-existing CT finding of BAF (3, 6, 7) and false positive Positron emission tomography with 2-[18F] fluoro-2-deoxy-D-glucose as a tracer (FDG-PET) uptake is also frequently found in anthracosis. These finding often mimic a metastatic lymphadenopathy seen in lung cancer (8-11).
The aim of this study is to define the clinical significance of BAF and its role in the prediction of metastatic lymphadenopathy in lung cancer patients.
This study was a retrospective review of a prospectively maintained research database of "Bronchoscopy of the Asan Medical Center" in Seoul, Korea. We selected patients who underwent EBUS-TBNA and diagnosed with lung cancer from May 2009 to August 2011. The diagnosis of lung cancer was made by pathological evidences; and the purpose of EBUS-TBNA was to assess a mediastinal staging (stage I to IIIB) or tissue confirmation (stage IV), like other institutes (2).
Their demographic features and clinical data were retrieved from the database and their electronic medical records (EMR). Demographic features included age, sex, clinical stage of lung cancer, target LNs, size of LNs, pathological findings of LNs from EBUS-TBNA and/or surgery, image findings of chest CT, bronchoscopy, EBUS-TBNA, and FDG-PET. BAF on bronchosocpy, aspirated tissue pigmentation for EBUS-TBNA, and standardized uptake value (SUV) for FDG-PET were checked. Lung cancer stage was determined according to TNM 7th edition. BAF and pigmentation of the EBUS-TBNA tissue were evaluated macroscopically by bronchoscopists (4) and the representative images are shown in Fig. 1A (bronchial anthracofibrosis) and 1B (macroscopic pigmentation of the EBUS-TBNA tissue).
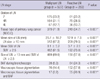
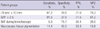
The clinical characteristics of total of 536 lung cancer patients are shown in Table 1. Of the 536 patients, 110 patients underwent surgical resection. Mean age was 64 yr (range: 29-85 yr), and 415 patients (77.4%) were male. Of these patients, 245 patients were diagnosed with adenocarcinoma (45.7%), 143 patients with squamous cell carcinoma (26.7%), 109 patients with small cell carcinoma (20.3%), 35 patients with other forms of non-small cell lung cancer (6.5%), and 4 patients with a combination of small cell carcinoma/non-small cell lung cancer (0.7%). BAF was observed in 55 patients (10.3%).
The characteristics of total 790 LNs are shown in Table 2. Malignant LN or reactive LN in EBUS-TBNA was defined according to the pathologic findings of biopsy or aspiration tissue. Of them, 527 LNs were malignant (66.7%) and 263 LNs were reactive (33.3%). Among the 263 reactive LNs, 69 LNs were resected by surgery and 92.8% of resected LNs were confirmed as reactive. The most frequently examined LN included 4R (30.3%), 7 (29.9%), and 4L (13.0%). The mean size of LN was 18.1 ± 10.0 mm and mean SUV was 6.6 ± 4.9. Pigmentation of EBUS-TBNA tissue was observed in 228 patients (28.9%) and BAF was observed in 92 LNs (11.6%) during bronchoscopy.
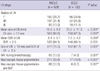
The comparison of LN characteristics between malignant LN and reactive LN is shown in Table 3. The mean SUV of the malignant LN group was 8.1 ± 5.2, and the mean SUV of the reactive LN group was 3.7 ± 2.6 (mean difference: 4.4; P < 0.001). The mean size of the malignant LN group was 20.7 ± 10.2 mm, and the mean size of the reactive LN group was 12.9 ± 7.3 mm (mean difference: 7.9 mm; P < 0.001). Pigmentation of EBUS-TBNA tissue was observed in 14.4% of malignant LNs, which was significantly lower than that of reactive LNs, 57.8%. BAF was also observed less frequently in malignant LNs than in reactive LNs, 5.3% and 24.3%, respectively. More detailed analyses according to tissue type of primary lung cancer, BAF in malignant LNs or reactive LNs are shown in Tables 4, 5, 6.
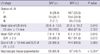
The relative risks for developing malignant LN were 3.3 (95% confidence interval [CI], 2.1-5.1; P < 0.001) for LN ≥ 1 cm in size, 1.8 (95% CI, 1.5-2.3; P < 0.001) for SUV ≥ 2.5, 0.4 (95% CI, 0.3-0.6; P < 0.001) for macroscopic tissue pigmentation, and 0.4 (95% CI, 0.3-0.6; P < 0.001) for BAF. There was no significant difference in terms of sex.
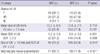
The adjusted odds ratio for malignant LN was 0.46 (95% CI, 0.26-0.81; P < 0.001) for BAF, and 0.22 (95% CI, 0.15-0.33; P < 0.001) for pigmentation of EBUS-TBNA tissue (Table 7) after adjusting an age, tissue type (NSCLC in contrast to SCLC), LN size (≥10 mm), and SUV (≥2.5). The specificities of pigmentation of EBUS-TBNA tissue and BAF for predicting a malignant LN were 42.2% and 75.7%, respectively, which was higher than the specificities of LN size ≥10 mm or SUV ≥2.5 (Table 8).
The effort to defining morphologic and sonographic features of EBUS has become more pronounced according to the increased performance of EBUS. Fujiwara et al. (12) described in 2010 that features of malignant LNs are round shape, distinct margin, heterogeneous echogenicity, and presence of coagulation necrosis sign. Thereafter, Nguyen et al. (13) reported EBUS digital images of lymph nodes can be used to differentiate malignant and benign lymphadenopathy. However, there are little reports about relationship between anthracofibrosis and metastatic LNs in EBUS-TBNA.
LN size and SUV on FDG-PET are known parameters for predicting metastatic lymphadenopathy in lung cancer patients (14). We analyzed the relative risk of LN size and SUV on FDG-PET for predicting malignant LN in lung cancer patients. As expected, an LN ≥10 mm in size tended to be malignant by 3.3-fold and SUV ≥2.5 by 2.5-fold. Also, the odds ratio of a larger LN (ie, LN ≥10 mm in size) developing malignancy was 9.99 compared with smaller LN with an odds ratio of 3.59 when the SUV was large (ie, SUV ≥2.5). The sensitivity of LN size and SUV for the diagnosis of malignant LN was excellent (>90%). However, their specificities were quite low: 20.5% and 27% for LN size and SUV, respectively.
BAF and pigmentation of aspirated tissue are observed easily during EBUS-TBNA, and those bronchoscopic findings can be identified by bronchoscopists without substantial error. However, their clinical implications are not appropriately explained during lung cancer work-up. Here, we showed higher specificities of BAF and macroscopic tissue pigmentation, 42.2% and 75.7%, respectively, than that of LN size and SUV despite of low sensitivities for the diagnosis of malignant LN. Multivariate analysis showed that the odds ratio of BAF and macroscopic tissue pigmentation was 0.46 and 0.22, respectively, which means that malignant LN shows less macroscopic tissue pigmentation and less BAF after consideration of age, tissue type, LN size, and SUV. According to the analyzed results, suggestive findings of malignant LNs are 1) younger age, 2) tissue type of SCLC, 3) LN size greater than 10 mm, 4) SUV greater than 2.5, 5) absence of BAF during bronchoscopy, and 6) absence of macroscopic tissue pigmentation during EBUS-TBNA.
There are some limitations about our study such as, retrospective design, absence of surgical confirmation of all LNs, and low sensitivity. In spite of these limitations, we try to attach a clinical implication to the BAF and macroscopic tissue pigmentation, which are occasionally inspected during EBUS-TBNA. Also, to the best of our knowledge, this is the first study to attach a clinical interpretation to BAF and tissue pigmentation in EBUS-TBNA.
Because Korea is an endemic area of tuberculosis, BAF is frequently observed and LN enlargement and false-positive FDG-PET SUV uptake mimic the mediastinal LN stage of lung cancer (8). We observed more frequent BAF or tissue pigmentation in reactive LN and proposed an additional interpretation of BAF and tissue pigmentation during EBNUS-TBNA. Putting BAF and macroscopic tissue pigmentation together with LN size and SUV, more reliable prediction would be possible before tissue confirmation.
Figures and Tables
Fig. 1
Endobronchial findings. (A) Bronchial anthracofibrosis. (B) Macroscopic tissue pigmentation observed during EBUS-TBNA.

References
1. Ettinger DS, Akerley W, Bepler G, Blum MG, Chang A, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Ganti AK, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2010. 8:740–801.
2. Lee BE, Kletsman E, Rutledge JR, Korst RJ. Utility of endobronchial ultrasound-guided mediastinal lymph node biopsy in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg. 2012. 143:585–590.
3. Gupta A, Shah A. Bronchial anthracofibrosis: an emerging pulmonary disease due to biomass fuel exposure. Int J Tuberc Lung Dis. 2011. 15:602–612.
4. Kim MA, Lee JC, Choi C. False-positive FDG-PET and bronchial anthracofibrosis. J Thorac Oncol. 2012. 7:1474.
5. Jang SJ, Lee SY, Kim SC, Lee SY, Cho HS, Park KH, Moon HS, Song JS, Park SH, Kim YK, et al. Clinical and radiological characteristics of non-tuberculous bronchial anthracofibrosis. Tuberc Respir Dis. 2007. 63:139–144.
6. Kim HY, Im JG, Goo JM, Kim JY, Han SK, Lee JK, Song JW. Bronchial anthracofibrosis (inflammatory bronchial stenosis with anthracotic pigmentation): CT findings. AJR Am J Roentgenol. 2000. 174:523–527.
7. Bilici A, Erdem T, Boysan SN, Acbay O, Oz B, Besirli K, Gundogdu S. A case of anthracosis presenting with mediastinal lymph nodes mimicking tuberculous lymphadenitis or malignancy. Eur J Intern Med. 2003. 14:444–446.
8. Kim YK, Lee KS, Kim BT, Choi JY, Kim H, Kwon OJ, Shim YM, Yi CA, Kim HY, Chung MJ. Mediastinal nodal staging of nonsmall cell lung cancer using integrated 18F-FDG PET/CT in a tuberculosis-endemic country: diagnostic efficacy in 674 patients. Cancer. 2007. 109:1068–1077.
9. Konishi J, Yamazaki K, Tsukamoto E, Tamaki N, Onodera Y, Otake T, Morikawa T, Kinoshita I, Dosaka-Akita H, Nishimura M. Mediastinal lymph node staging by FDG-PET in patients with non-small cell lung cancer: analysis of false-positive FDG-PET findings. Respiration. 2003. 70:500–506.
10. Lee SH, Min JW, Lee CH, Park CM, Goo JM, Chung DH, Kang CH, Kim YT, Kim YW, Han SK, et al. Impact of parenchymal tuberculosis sequelae on mediastinal lymph node staging in patients with lung cancer. J Korean Med Sci. 2011. 26:67–70.
11. Choi HY, Kim YK, Lee JJ, Kim SE. Bronchial anthracofibrosis: a potential false-positive finding on F-18 FDG PET. Ann Nucl Med. 2012. 26:681–683.
12. Fujiwara T, Yasufuku K, Nakajima T, Chiyo M, Yoshida S, Suzuki M, Shibuya K, Hiroshima K, Nakatani Y, Yoshino I. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: a standard endobronchial ultrasound image classification system. Chest. 2010. 138:641–647.
13. Nguyen P, Bashirzadeh F, Hundloe J, Salvado O, Dowson N, Ware R, Masters IB, Bhatt M, Kumar AR, Fielding D. Optical differentiation between malignant and benign lymphadenopathy by grey scale texture analysis of endobronchial ultrasound convex probe images. Chest. 2012. 141:709–715.
14. Silvestri GA, Tanoue LT, Margolis ML, Barker J, Detterbeck F. American College of Chest Physicians. The noninvasive staging of non-small cell lung cancer: the guidelines. Chest. 2003. 123:147S–156S.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download