Abstract
Although gemifloxacin has low in vitro activity against Mycobacterium tuberculosis, the effect of gemifloxacin on the delay of tuberculosis (TB) treatment has not been validated in a clinical setting. The study group included patients with culture-confirmed pulmonary TB who initially received gemifloxacin for suspected community-acquired pneumonia (CAP). Two control groups contained patients treated with other fluoroquinolones or nonfluoroquinolone antibiotics. Sixteen cases were treated with gemifloxacin for suspected CAP before TB diagnosis. Sixteen and 32 patients were treated with other fluoroquinolones and nonfluoroquinolones, respectively. The median period from the initiation of antibiotics to the administration of anti-TB medication was nine days in the gemifloxacin group, which was significantly different from the other fluoroquinolones group (35 days). The median times for the nonfluoroquinolone group and the gemifloxacin group were not significantly different. There were no significant differences between the gemifloxacin and other fluoroquinolone group in terms of symptomatic and radiographic improvements. However, the frequency of radiographic improvement in the other fluoroquinolones group tended to be higher than in the gemifloxacin group. Gemifloxacin might be the preferred fluoroquinolone for treating CAP, to alleviate any concerns about delaying TB treatment.
Tuberculosis (TB) is an important cause of morbidity and mortality worldwide, especially in Asia and Africa. Estimates of the global disease burden caused by TB during 2009 were 9.4 million incident cases, 14 million prevalent cases, and 1.7 million deaths (1). Delays in the diagnosis and treatment of tuberculosis are associated with increased morbidity and mortality because of Mycobacterium tuberculosis (2-4).
Fluoroquinolones have been widely used to treat pneumonia, including community-acquired pneumonia (CAP), because they have excellent activity against a wide variety of respiratory tract pathogens (5). The antimicrobial activities of respiratory quinolones also extend to M. tuberculosis, and they are recommended as part of drug regimens for treating rifampin-resistant TB (6, 7). However, the anti-TB activity of respiratory quinolones may cause problems during the treatment of CAP in TB endemic areas because fluoroquinolones can mask TB, which might be associated with delays in the diagnosis and treatment of TB (4, 8-11). Quinolones also have different in vitro anti-TB activities. The fourth-generation fluoroquinolone, gemifloxacin, retains the antibacterial activity spectrum of classic fluoroquinolones, but it has a relatively low in vitro activity against M. tuberculosis (12-14). However, the clinical implication of using quinolones with different in vitro activities against M. tuberculosis has not been validated in terms of their masking of TB. Based on in vitro evidence that gemifloxacin might not mask TB during the treatment of CAP, this study was performed to evaluate the clinical effects of gemifloxacin on delaying the treatment of TB compared with other quinolone and nonquinolone antibiotics that are used for treating CAP.
This study included adult patients aged over 18 yr who had been treated with antibiotics for more than three consecutive days after an initial assessment of CAP followed by a later diagnosis of pulmonary TB. Patients were recruited from two hospitals affiliated with Seoul National University, i.e., Seoul National University Hospital and Seoul National University Bundang Hospital. TB was confirmed microbiologically by culture studies from January 2003 to December 2010. We excluded subjects who were treated with antibiotics such as amoxicillin, which have definite or uncertain anti-TB activity (15, 16).
The case group was defined as patients who had been treated with gemifloxacin prior to the administration of anti-TB drugs, while the control groups included patients who were treated initially with other fluoroquinolones or nonfluoroquinolone antibiotics. Controls were matched for age and sex in each case group. The symptoms and pharmaceutical records were reviewed for each patient using their medical records. Serial radiographs were also reviewed.
We determined the time intervals from the initiation of antibiotics until the administration of anti-TB medications, as well as any symptomatic improvements and the time when any radiologic changes were apparent after treatment with gemifloxacin, other fluoroquinolone, and nonfluoroquinolone group. Symptomatic improvement was recorded when more than one of the following symptoms was improved: febrile sensation, coughing, dyspnea, general weakness, and pleuritic chest pain. Radiographic improvement was defined when radiographic improvement was detected in a follow-up chest radiograph within 1-4 weeks of antibiotic treatment.
Statistical analysis was performed using the software package SPSS® (Version 17.0, Chicago, IL, USA). Comparisons among groups were conducted using Fisher's exact test for categorical variables. For continuous variables, the Mann-Whitney U-test was applied to make comparisons between two groups, and the Kruskal-Wallis test among three groups. Statistical significance was determined at P < 0.05.
Sixteen patients were treated with gemifloxacin for suspected bacterial CAP prior to their diagnosis with TB. Sixteen and 32 patients were treated with fluoroquinolones other than gemifloxacin and nonfluoroquinolone antibiotics, respectively, and were matched for age and sex. Their median age was 63 yr, and 48 (75%) were male (Table 1). The clinical characteristics of the cases and controls are summarized in terms of their symptoms in Table 2, and their radiographic results in Table 3. There were no differences in the symptomatic and radiographic characteristics among the three groups.
The median time interval from the initiation of antibiotics until the administration of anti-TB medications was nine days (range, 7-72 days) in the gemifloxacin group, which was significantly less than the other fluoroquinolones group (median, 35 days; range, 14-72 days; P = 0.012) (Fig. 1). The median time interval for the nonfluoroquinolone group was 7 days (range, 3-63 days), which was not significantly different from the gemifloxacin group (P = 0.148).
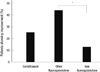
The proportions of subjects with symptomatic improvements did not differ statistically among the three groups. However, the symptoms of patients appeared to be relieved more frequently when treated with fluoroquinolones rather than nonfluoroquinolones (Fig. 2).
Seven patients (44%) in the other fluoroquinolones group had improved follow-up radiographs, while four patients (25%) had improvements in the gemifloxacin group. The proportion of patients with radiographic improvements did not differ between the gemifloxacin and nonfluoroquinolones groups (Fig. 3). However, the frequency of radiographic improvement in the other fluoroquinolones group tended to be higher than in the gemifloxacin group. Radiographic improvement was observed in only 12% of patients treated with nonfluoroquinolone antibiotics.
Even after adjusting with age, sex, and the duration of antibiotics, the impact of antibiotics group in order of other fluoroquinolones, gemifloxacin, and non-fluoroquinolone antibiotics on the frequency of radiographic improvement was statistically significant (P = 0.036) as well as the delay in the administration of anti-TB medication (P = 0.037).
This study aimed to elucidate whether the different in vitro activity of gemifloxacin against M. tuberculosis compared with other fluoroquinolones was replicated in clinical settings in a country where TB is endemic. This study investigated the clinical effects of gemifloxacin on delaying TB treatment to determine whether they were different from other fluoroquinolones. Gemifloxacin was similar to nonfluoroquinolone antibiotics in terms of the time interval between the initial antibiotic treatment and the administration of anti-TB medications.
The results of this study are expected to have several clinical implications. The primary outcomes of this study demonstrated that there was no difference in terms of the antimycobacterial activity of gemifloxacin between the results of the in vitro study and those obtained in a human population. Fluoroquinolones are known to mask TB, which might cause delays in its diagnosis and treatment (4, 8-10). Thus, clinicians in areas where TB is endemic may be reluctant to prescribe respiratory fluoroquinolones to treat suspected pneumonia (8, 9). This clinical validation of the relatively low activity of gemifloxacin against M. tuberculosis might help alleviate the concern of clinicians that it could delay TB treatment, especially in areas where TB is endemic.
Previous studies have reported differences among the different classes of fluoroquinolones in terms of their in vitro antimicrobial activity against M. tuberculosis (12, 13, 17). Ruiz-Serrano et al. (12) tested the susceptibility of 250 M. tuberculosis isolates to ciprofloxacin, ofloxacin, levofloxacin, and gemifloxacin. Levofloxacin, ciprofloxacin, and ofloxacin had good activity (minimal inhibitory concentration, MIC) in 90% of the strains tested (MIC90=1-2 µg/mL), whereas gemifloxacin had lower in vitro activity, with an MIC90 of 8 µg/mL. Tan et al. reported that the MICs of fluoroquinolones tested against multidrug-resistant (MDR) TB and non-MDR TB isolates increased in the order: moxifloxacin, levofloxacin, ciprofloxacin, and gemifloxacin. Among the fluoroquinolones tested, levofloxacin and moxifloxacin had the lowest MICs with M. tuberculosis and, in most cases, the levofloxacin and moxifloxacin MICs were at least 16 times lower than those of gemifloxacin (13). These differences may be attributable to the quinolone structure-activity relationship. The two major groups of the quinolones are classified according to their basic structure, i.e., quinolones and naphthyridones. The presence of a nitrogen at position 8 characterizes naphthyridones, whereas a carbon and an associated group at position 8 characterizes quinolones (18). The naphthyridone structure of gemifloxacin was shown to be a negative factor in a quantitative study of its structure-activity relationship with antimycobacterial activity (17), which might explain its poor anti-TB activity. Additional differences in the mode of inhibition of their targets by quinolones might explain their variable activity. The activity of quinolones is believed to target DNA gyrase in M. tuberculosis, which results in the inhibition of DNA supercoiling and the disruption of DNA replication. Gemifloxacin had a low inhibitory activity against M. tuberculosis DNA gyrase, with 50% inhibitory concentrations against DNA gyrase of >10 µg/mL (19).
However, the results of in vitro susceptibility testing are not always replicated in real clinical settings. The MIC for penicillin-resistant Streptococcus pneumoniae may also fail to elicit an adequate therapeutic response in cases of pneumonia (20, 21). Therefore, it is mandatory to validate the in vitro response of the human population to determine the clinical application spectrum of new drugs. The results of the current study showed that there was no disparity between the in vitro results and those obtained in a human population in terms of the antimycobacterial activity of gemifloxacin.
We evaluated the effects of antibiotics on clinical outcomes in terms of improved symptoms and radiographic findings as well as the time delay until the administration of anti-TB medication. The radiographic findings indicated major improvements in all three groups after treatment with nongemifloxacin fluoroquinolones, which may delay the treatment of pulmonary TB. Therefore, this may merit concern about the masking of pulmonary TB in CAP patients treated with nongemifloxacin fluoroquinolones. These findings are consistent with previous reports (4, 8-10). However, there were no statistical differences in the symptomatic and radiographic improvements in CAP with gemifloxacin compared with nonquinolone antibiotics.
Because the antimycobacterial activity of antibiotics increases in strength in the order: nongemifloxacin fluoroquinolones, gemifloxacin, and nonquinolone antibiotics, there was no time delay in the gemifloxacin group than nongemifloxacin fluoroquinolones group.
This study reports clinically significant findings, but several limitations need to be considered. First, only a limited number of pulmonary TB cases were treated with gemifloxacin during recent clinical practice, which means that the small sample size may have failed to detect any differences between gemifloxacin and other respiratory quinolones in terms of symptomatic and radiographic improvements. The retrospective study design also had limitations in explaining the overall story of gemifloxacin efficacy against pulmonary TB. Therefore, questions remain about the safe treatment duration for gemifloxacin without masking TB, and any different clinical effects of gemifloxacin on extrapulmonary TB or MDR TB, which should be addressed by further prospective studies.
Gemifloxacin has low activity against Mycobacterium tuberculosis in clinical setting. Therefore gemifloxacin might be the preferred fluoroquinolone for treating CAP, to alleviate any concerns about masking the diagnosis or delaying the treatment of pulmonary TB in countries where TB is endemic.
Figures and Tables
Fig. 1
The time interval from the initiation of antibiotics until the administration of anti-TB drugs. The median time intervals from the initiation of antibiotics until the administration of anti-TB medications were nine days (range, 7-72 days) in the gemifloxacin group, 35 days in the other fluoroquinolones group (range, 14-72 days) and 7 days in the nonfluoroquinolone group (range, 3-63 days). *P value < 0.05.

Fig. 2
Differences in the proportions of patients with symptomatic improvements. Symptomatic improvement was defined when more than one symptom of the followings was improved: febrile sense, cough, dyspnea, general weakness and pleuritic chest pain. The proportions of subjects with symptomatic improvements were 50% in the gemifloxacin group and the other fluoroquinolones group and 31% in the nonfluoroquinolone group.
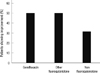
Fig. 3
Differences in the proportions of patients with radiographic improvement. Radiographic improvement was defined when radiograhic improvement was detected on a follow-up chest radiograph within 1-4 weeks after antibiotic treatment. Seven patients (44%) in the other fluoroquinolones group had improved follow-up radiographs, while four patients (25%) had improvements in the gemifloxacin group. Radiographic improvement was observed in only 12% of patients treated with nonfluoroquinolone antibiotics. *P value < 0.05.
References
1. WHO global tuberculosis control report 2010: summary. Cent Eur J Public Health. 2010. 18:237.
2. Pablos-Méndez A, Sterling TR, Frieden TR. The relationship between delayed or incomplete treatment and all-cause mortality in patients with tuberculosis. JAMA. 1996. 276:1223–1228.
3. Sherman LF, Fujiwara PI, Cook SV, Bazerman LB, Frieden TR. Patient and health care system delays in the diagnosis and treatment of tuberculosis. Int J Tuberc Lung Dis. 1999. 3:1088–1095.
4. Wang JY, Hsueh PR, Jan IS, Lee LN, Liaw YS, Yang PC, Luh KT. Empirical treatment with a fluoroquinolone delays the treatment for tuberculosis and is associated with a poor prognosis in endemic areas. Thorax. 2006. 61:903–908.
5. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007. 44:S27–S72.
6. Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003. 167:603–662.
7. Lew WJ. The up-to-date of anti-tuberculosis treatment. Tuberc Respir Dis. 2006. 60:270–276.
8. Yoon YS, Lee HJ, Yoon HI, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. Impact of fluoroquinolones on the diagnosis of pulmonary tuberculosis initially treated as bacterial pneumonia. Int J Tuberc Lung Dis. 2005. 9:1215–1219.
9. Jeon CY, Calver AD, Victor TC, Warren RM, Shin SS, Murray MB. Use of fluoroquinolone antibiotics leads to tuberculosis treatment delay in a South African gold mining community. Int J Tuberc Lung Dis. 2011. 15:77–83.
10. Dooley KE, Golub J, Goes FS, Merz WG, Sterling TR. Empiric treatment of community-acquired pneumonia with fluoroquinolones, and delays in the treatment of tuberculosis. Clin Infect Dis. 2002. 34:1607–1612.
11. Lee HS, Kang YA, Oh JY, Lee JH, Yoo CG, Lee CT, Kim YW, Han SK, Shim YS, Yim JJ. A case of pulmonary tuberculosis with delayed diagnosis due to the temporary clinical improvement after use of levofloxacin and amikacin under the impression of community acquired pneumonia. Tuberc Respir Dis. 2003. 55:395–401.
12. Ruiz-Serrano MJ, Alcalá L, Martínez L, Díaz M, Marín M, González-Abad MJ, Bouza E. In vitro activities of six fluoroquinolones against 250 clinical isolates of Mycobacterium tuberculosis susceptible or resistant to first-line antituberculosis drugs. Antimicrob Agents Chemother. 2000. 44:2567–2568.
13. Tan CK, Lai CC, Liao CH, Chou CH, Hsu HL, Huang YT, Hsueh PR. Comparative in vitro activities of the new quinolone nemonoxacin (TG-873870), gemifloxacin and other quinolones against clinical isolates of Mycobacterium tuberculosis. J Antimicrob Chemother. 2009. 64:428–429.
14. Lai CC, Tan CK, Huang YT, Chou CH, Hung CC, Yang PC, Luh KT, Hsueh PR. Extensively drug-resistant Mycobacterium tuberculosis during a trend of decreasing drug resistance from 2000 through 2006 at a Medical Center in Taiwan. Clin Infect Dis. 2008. 47:e57–e63.
15. Nadler JP, Berger J, Nord JA, Cofsky R, Saxena M. Amoxicillin-clavulanic acid for treating drug-resistant Mycobacterium tuberculosis. Chest. 1991. 99:1025–1026.
16. Chambers HF, Kocagöz T, Sipit T, Turner J, Hopewell PC. Activity of amoxicillin/clavulanate in patients with tuberculosis. Clin Infect Dis. 1998. 26:874–877.
17. Jacobs MR. Activity of quinolones against mycobacteria. Drugs. 1995. 49:67–75.
18. Moadebi S, Harder CK, Fitzgerald MJ, Elwood KR, Marra F. Fluoroquinolones for the treatment of pulmonary tuberculosis. Drugs. 2007. 67:2077–2099.
19. Aubry A, Pan XS, Fisher LM, Jarlier V, Cambau E. Mycobacterium tuberculosis DNA gyrase: interaction with quinolones and correlation with antimycobacterial drug activity. Antimicrob Agents Chemother. 2004. 48:1281–1288.
20. Cardoso MR, Nascimento-Carvalho CM, Ferrero F, Berezin EN, Ruvinsky R, Camargos PA, Sant'anna CC, Brandileone MC, de Fátima P, March M, et al. Penicillin-resistant pneumococcus and risk of treatment failure in pneumonia. Arch Dis Child. 2008. 93:221–225.
21. Friedland IR, Klugman KP. Antibiotic-resistant pneumococcal disease in South African children. Am J Dis Child. 1992. 146:920–923.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download