Abstract
Evaluating predictive factors for high-risk adenomas at the third colonoscopy based on two prior colonoscopies may help evaluate high-risk adenoma at the third colonoscopy. We analyzed clinical data of 131 patients at Severance Hospital from January 1997 to January 2011. All of them underwent two subsequent colonoscopies after removal of adenomas during an initial colonoscopy. Among 20 patients with high-risk adenoma at the first and second colonoscopies, 10 (50%) patients had high-risk adenoma at the third colonoscopy. Among the 67 patients who had high-risk adenoma only once at the first or second colonoscopy, 15 (22.4%) patients had high-risk adenoma at the third colonoscopy but among the 44 patients without high-risk adenoma at the first and second colonoscopies, only 1 (2.3%) patient had high-risk adenoma at the third colonoscopy (P < 0.001). A multivariate time dependent covariate Cox regression analysis confirmed that high-risk adenoma at the first and/or second colonoscopy (HR, 9.56; 95% CI, 2.37-38.54; P = 0.002) was independent predictor of high-risk adenoma at the third colonoscopy. Given these findings, data from two prior colonoscopies, not one prior examination, may help identify high-risk populations at the third colonoscopy who require careful colonoscopic surveillance.
Surveillance colonoscopy plays an important role in the early detection of colon polyps and performing subsequent polypectomy, which has been shown to reduce the rate of colorectal cancer development (1). Despite a complete initial polypectomy, 37%-60% of the patients are found to have recurrent polyps during subsequent examinations (2, 3). According to the current practice guidelines for determining the optimal follow-up intervals in patients with adenoma (4-6), a subsequent surveillance colonoscopy is recommended at ten years for patients without adenoma; between five to ten years for those with low-risk adenomas, defined as one or two small (<10 mm) adenomas; and at three years for those with high-risk adenomas defined as advanced adenomas (tubular adenoma≥1 cm, adenoma with a villous or tubulovillous component, a lesion with high-grade dysplasia or adenocarcinoma) or ≥ three synchronous adenomas (6). However, the optimal recommended surveillance interval for the third colonoscopy after initial adenoma removal has not been established. The findings of the third colonoscopy could be affected by the results of both the first and second colonoscopies. Here, we evaluated risk factors for the presence of high-risk adenomas at the time of a third colonoscopy based on the findings from two prior colonoscopic examinations, which will help determine optimal colonoscopic intervals by identifying high-risk patients that require careful colonoscopic surveillance using a time dependent covariate Cox regression analysis.
Between January 1997 and February 2011, our retrospective cohort included a total of 212 patients who underwent two consecutive surveillance colonoscopies after an initial polyp removal (size ≥ 5 mm and number ≥ 1) at the first colonoscopy at a single tertiary academic medical center. Of these, 23 patients with hyperplastic polyps or benign mucosal lesions, 30 patients with colon cancer, 11 patients with inflammatory bowel disease and 17 patients whose surveillance colonoscopy interval was ≤ 6 months were excluded from this study. We therefore analyzed the clinical and colonoscopic data of the remaining 131 patients. Colonoscopic data were collected at the first, second, and third colonoscopy. Clinical data of each patient including age, sex, body mass index (BMI), and serum levels of albumin, cholesterol, glucose, and bilirubin were collected at the first colonoscopy.
All the examinees followed standard methods of bowel preparation. For the colonoscopy arranged at the morning, examinees should drink the 4 L of colyte at the night of one day before colonoscopy. For the colonoscopy arranged at the afternoon, examinees should drink the 2 L of colyte at the night of one day before colonoscopy and remaining 2 L of colyte at the morning of examination day. Experienced endoscopists, all of whom had performed over 1,000 colonoscopies, conducted all examinations using a standard colonoscope (CF Q240L, CF Q240I, CF H260AI, or CF Q260AI; Olympus Optical Co., Ltd., Tokyo, Japan), and the number, size, and location of all polyps identified were recorded during the procedure at the first, second, and third colonoscopy. Moreover, all polyps were categorized histologically according to the World Health Organization (WHO) classification system (7). The right colon was defined as the cecum, ascending colon, hepatic flexure, and the transverse colon, whereas the left colon included the splenic flexure, descending colon, sigmoid colon, and the rectum. All polyps less than 5 mm were removed with cold biopsy forceps, while polyps larger than 5 mm were removed by either endoscopic mucosal resection or snare polypectomy. Large sessile or flat adenomas greater than 20 mm in size were resected by piecemeal endoscopic mucosal resection or endoscopic submucosal dissection (8, 9).
Continuous variables were compared via ANOVA, while categorical data were analyzed using the chi-square test. All statistical analyses were performed using SPSS Statistics (version 18.0.0, IBM Corp., Armonk, NY, USA). Time dependent covariate Cox regression analysis (SAS version 9.2 Institute Inc., Cary, NC, USA) using colonoscopic data collected at the first and second colonoscopy was employed to identify time dependent covariates predictive of high-risk adenoma at the third colonoscopy. Cox regression analysis was used to identify clinical covariates predictive of high-risk adenoma at the third colonoscopy. In all cases, P values <0.05 were considered statistically significant.
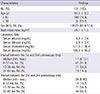
Two subsequent surveillance colonoscopies were performed in 131 patients after adenoma (size ≥ 5 mm and number ≥ 1) removal at the first colonoscopy. The baseline characteristics of the study population were summarized in Table 1. Their mean age was 65.5 ± 8.5 yr. The mean body mass index and serum levels of albumin, bilirubin, and cholesterol were 24.1 ± 1.8 kg/m2, 4.4 ± 0.4 mg/dL, 0.8 ± 0.4 mg/dL, and 177.9 ± 38.4 mg/dL, respectively. The median interval (min-max) between the first and second colonoscopy was 17 (6-101) months, while the median interval (min-max) between the second and third colonoscopy was 24 (6-90) months.
In Fig. 1, there were 131 (100%), 81 (61.8%), and 76 (58.1%) patients in whom adenomas were detected at the first, second, and third colonoscopies, respectively (P < 0.001). The numbers of patients with high-risk adenomas were 77 (58.8%), 30 (22. 9%), and 26 patients (19.8%) at the first, second, and third colonoscopies, respectively (P < 0.001) (Fig. 1). The endoscopic outcomes at the first, second, and third colonoscopies are summarized in Table 2. The mean numbers of advanced adenomas at the time of the first, second, and third colonoscopies were 1.5 ± 0.9, 1.1 ± 0.3, and 1.0 ± 0.0, respectively (P = 0.016). The numbers of adenomas that were either high-grade dysplasia or adenocarcinoma at the first, second, and third colonoscopies were 10 (12.3%), 0 (0%), and 2 (12.5%), respectively (P < 0.001). There were 17 (21.0%), 5 (22.7%), and 0 (0%) villous or tubulovillous adenomas at the first, second, and third colonoscopies, respectively (P < 0.001). The numbers of tubular adenomas ≥ 10 mm at the first, second, and third colonoscopies were 54 (66.7%), 17 (77.3%), and 14 (87.5%), respectively (P < 0.001). There was no significant difference in Ottawa scores, withdrawal times, and cecal intubation rates between the first, second, and third colonoscopies.
In Fig. 2, among the 20 patients with high-risk adenoma at the first and second colonoscopy, 10 (50%) patients had high-risk adenoma at the third colonoscopy. Among the 67 patients who had high-risk adenoma only once at the first or second colonoscopy, 15 (22.4%) patients had high-risk adenoma at the third colonoscopy. However, among the 44 patients without any high-risk adenoma at the first and second colonoscopy, only 1 (2.3%) patient had high-risk adenoma at the third colonoscopy (P < 0.001).
Among the 30 patients with high-risk adenoma at the second colonoscopy, 10 out of 20 patients (50%) with high-risk adenoma at the first colonoscopy had high-risk adenoma at the third colonoscopy, and 5 out of 10 patients (50%) without high-risk adenoma at the first colonoscopy had high-risk adenoma at the third colonoscopy (P > 0.999).
In the absence of high-risk adenoma at the second colonoscopy, there was a higher incidence of high-risk adenoma at the third colonoscopy in presence of high-risk adenoma at the first colonoscopy than absence of high-risk adenoma at the first colonoscopy (17.5% (10/57) vs 2.3% (1/44), P = 0.021).
According to the univariate and multivariate time dependent covariate Cox regression analysis, a high-risk adenoma at the first or second colonoscopy was only independent predictor of high-risk adenoma at the third colonoscopy (hazard ratio [HR], 9.56; 95% confidential interval [CI], 2.37-38.54; P = 0.002) (Table 3). Upon Cox regression analysis, age, gender, BMI, serum levels of albumin, cholesterol, glucose, bilirubin, Ottawa score, and withdrawal time (min) were not significantly related to recurrence of high-risk adenoma at the third colonoscopy.
The findings of the third colonoscopy could be affected by the results of both the first and second colonoscopies. Evaluating the risk factors based on the first and second colonoscopies for high-risk adenoma recurrence at the third colonoscopy would give better information than if based on the only one prior colonoscopy. Here we evaluated the factors predictive of high-risk adenomas at the third colonoscopy based on the findings from two prior colonoscopic examination by considering the concept of time. In our study the patients with high-risk adenoma at the first and/or second colonoscopy had increased risk of recurred high-risk adenoma at the third colonoscopy than the patients without high-risk adenoma at the first and second colonoscopy (HR, 9.56; 95% CI, 2.37-38.54; P = 0.002).
Our results also showed that among the patients who had high-risk adenoma at the second colonoscopy, the presence of high-risk adenoma at the first colonoscopy did not have a significant effect on the recurrence of high-risk adenoma at the third colonoscopy. However, even if the patients did not have high-risk adenoma at the second colonoscopy, the presence of high-risk adenoma at the first colonoscopy had a significant effect on the recurrence of high-risk adenoma at the third colonoscopy. Therefore, the patients without high-risk adenoma at the second colonoscopy should be divided according to the finding of first colonoscopy for evaluating recurrence risk of high-risk adenoma at the third colonoscopy. Then patients with high-risk adenoma at the first colonoscopy may need more careful surveillance with shorter intervals than the patients without high-risk adenoma at the first colonoscopy. Likewise, if the patients underwent multiple colonoscopies, all data from previous colonoscopic findings would be checked for planning the surveillance schedule. Even if the results of the most recent colonoscopy were unremarkable, the earlier results of colonoscopy should be considered to distinguish the high-risk patients.
According to a previous prospective cohort study of patients undergoing multiple surveillance colonoscopies, the result of the first colonoscopy had no significant effect on the recurrence of high-risk adenoma at the third colonoscopy if there was high-risk adenoma at the second colonoscopy (18.2% of patients with high-risk adenoma at the first colonoscopy vs 20% of patients with low-risk adenoma found at the first colonoscopy (P = 0.780) which was similar to our findings (10). In another study, 15% of patients with normal findings at second colonoscopy and 40% of patients with a neoplasia at the second colonoscopy had a neoplasia at a subsequent examination (11). In our results, 10.9% (11/101) of patients without high-risk adenoma at the second colonoscopy and 50% (15/30) of patients with high-risk adenoma at second colonoscopy had high-risk adenoma at the third colonoscopy.
To date, various risk factors have been identified in prior studies as predictors for recurrent adenomas or advanced adenomas, including size (12), number (13), histology (12), and advanced adenomas (13-19). Nonetheless, other studies suggested that the number of adenomas (14, 15, 20), polyp size (20-22), and histology (14, 20) were not related to advanced adenomas at follow-up colonoscopy. As yet the data regarding the individual predictive factors of high-risk adenomas are inconsistent. Such results imply that no conclusive single characteristics predict the recurrence of high-risk adenoma, especially in the setting of multiple surveillance colonoscopies.
There were important strengths of our study. First, in this study we evaluated the presence of high-risk adenoma from two prior colonoscopic examinations as a risk factor of recurrence of high-risk adenoma at the third colonoscopy. The meaning of high-risk adenoma included size, number, and histology of polyps. We evaluated the high-risk adenoma as risk factor to overcome these individual inconclusive characteristics predictive of high-risk adenoma recurrence in previous studies (12-14, 20-22). The second strength of our study lies with the multivariate time dependent covariate Cox regression analysis of data culled from two prior examinations. The time dependent covariate Cox regression model is used as a method for analyzing time-to-event data, as it accounts for multiple covariates with values that change according to time and treats time as a functioning factor. Our study was unique in using time dependent covariate Cox regression model to analyze risk factor of recurrence of high-risk adenoma in multiple colonoscopies. Third, despite the nature of this retrospective study, we sought to identify specific predictive factors for high-risk adenomas at subsequent surveillance colonoscopies by analyzing well-organized electronic medical database from two prior colonoscopies. However, there were limiting points of interpretation for our study. First, the number of enrolled examiners (n=131) was relatively small, and selection bias was inevitably existed because the patients were enrolled retrospectively from a tertiary medical center. Second, the median interval between sequential colonoscopies was shorter than recommended intervals of practice guidelines. The reason why the patients in this study underwent repeat colonoscopies at these shorter intervals may be explained by poor bowel preparation. Mean values of Ottawa score at the first, second and third colonoscopy were 7.8 ± 2.4, 7.7 ± 2.3, and 7.3 ± 2.1. The polyps found at follow up colonoscopy could be a missed lesion or synchronous lesions at previous colonoscopy. Third, even though the short median interval between sequential colonoscopies, the recurrent rates of adenoma and high risk adenoma were high in this study. This might be caused by previous not enough clean bowel preparation for detection of adenomas.
In conclusion, our results indicated that the patients with high-risk adenoma at the first and/or second colonoscopy had increased risks of high-risk adenoma at the third colonoscopy compared to the patients without high-risk adenoma at first and second colonoscopies. Given these findings, data from two prior colonoscopies, not one prior examination, may help identify high-risk populations at the third colonoscopy who require careful colonoscopic surveillance.
Figures and Tables
Fig. 1
The numbers of patients with any adenoma and high-risk adenoma at the first, second, and third colonoscopies. *P values < 0.05 compared with the numbers of patients with any adenoma at the first colonoscopy; †P values < 0.05 compared with the numbers of patients with high-risk adenoma at the first colonoscopy.
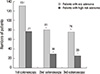
Fig. 2
The outcomes of the first, second, and third colonoscopies of patients with and without high-risk adenoma.
References
1. Fukutomi Y, Moriwaki H, Nagase S, Tajika M, Naito T, Miwa Y, Yamada Y, Araki H, Okuno M, Nagura K, et al. Metachronous colon tumors: risk factors and rationale for the surveillance colonoscopy after initial polypectomy. J Cancer Res Clin Oncol. 2002; 128:569–574.
2. Yood MU, Oliveria S, Boyer JG, Wells K, Stang P, Johnson CC. Colon polyp recurrence in a managed care population. Arch Intern Med. 2003; 163:422–426.
3. Neugut AI, Jacobson JS, Ahsan H, Santos J, Garbowski GC, Forde KA, Treat MR, Waye J. Incidence and recurrence rates of colorectal adenomas: a prospective study. Gastroenterology. 1995; 108:402–408.
4. Atkin WS, Saunders BP. British Society for Gastroenterology. Association of Coloproctology for Great Britain and Ireland. Surveillance guidelines after removal of colorectal adenomatous polyps. Gut. 2002; 51:V6–V9.
5. Davila RE, Rajan E, Baron TH, Adler DG, Egan JV, Faigel DO, Gan SI, Hirota WK, Leighton JA, Lichtenstein D, et al. ASGE guideline: colorectal cancer screening and surveillance. Gastrointest Endosc. 2006; 63:546–557.
6. Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O'brien MJ, Levin B, Smith RA, Lieberman DA, Burt RW, Levin TR, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. 2006; 56:143–159.
7. Hamilton SR, Aaltonen LA. World Health Organization classification of tumours: pathology and genetics of tumours of the digestive system. Lyon: IARC Press;2000.
8. Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010; 72:1217–1225.
9. Tanaka S, Oka S, Chayama K. Colorectal endoscopic submucosal dissection: present status and future perspective, including its differentiation from endoscopic mucosal resection. J Gastroenterol. 2008; 43:641–651.
10. Robertson DJ, Burke CA, Welch HG, Haile RW, Sandler RS, Greenberg ER, Ahnen DJ, Bresalier RS, Rothstein RI, Cole B, et al. Using the results of a baseline and a surveillance colonoscopy to predict recurrent adenomas with high-risk characteristics. Ann Intern Med. 2009; 151:103–109.
11. Blumberg D, Opelka FG, Hicks TC, Timmcke AE, Beck DE. Significance of a normal surveillance colonoscopy in patients with a history of adenomatous polyps. Dis Colon Rectum. 2000; 43:1084–1091.
12. Bertario L, Russo A, Sala P, Pizzetti P, Ballardini G, Andreola S, Spinelli P. Predictors of metachronous colorectal neoplasms in sporadic adenoma patients. Int J Cancer. 2003; 105:82–87.
13. Noshirwani KC, van Stolk RU, Rybicki LA, Beck GJ. Adenoma size and number are predictive of adenoma recurrence: implications for surveillance colonoscopy. Gastrointest Endosc. 2000; 51:433–437.
14. Martínez ME, Sampliner R, Marshall JR, Bhattacharyya AK, Reid ME, Alberts DS. Adenoma characteristics as risk factors for recurrence of advanced adenomas. Gastroenterology. 2001; 120:1077–1083.
15. Lorenzo-Zúñiga V, Moreno de Vega V, Domènech E, Mañosa M, Cabré E, Planas R, Boix J. High-definition colonoscopy and risk factors for recurrence of advanced adenomas in patients with a personal history of polyps. Eur J Gastroenterol Hepatol. 2011; 23:425–430.
16. Lieberman DA, Weiss DG, Harford WV, Ahnen DJ, Provenzale D, Sontag SJ, Schnell TG, Chejfec G, Campbell DR, Kidao J, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007; 133:1077–1085.
17. Nusko G, Hahn EG, Mansmann U. Characteristics of metachronous colorectal adenomas found during long-term follow-up: analysis of four subsequent generations of adenoma recurrence. Scand J Gastroenterol. 2009; 44:736–744.
18. Jørgensen OD, Kronborg O, Fenger C, Rasmussen M. Influence of long-term colonoscopic surveillance on incidence of colorectal cancer and death from the disease in patients with precursors (adenomas). Acta Oncol. 2007; 46:355–360.
19. Moon CM, Cheon JH, Choi EH, Kim ES, Park JJ, Han SY, Kim DH, Kim TI, Kim WH. Advanced synchronous adenoma but not simple adenoma predicts the future development of metachronous neoplasia in patients with resected colorectal cancer. J Clin Gastroenterol. 2010; 44:495–501.
20. Külling D, Christ AD, Karaaslan N, Fried M, Bauerfeind P. The presence of more than two index adenomas is the strongest predictor of metachronous colon adenomas. Swiss Med Wkly. 2002; 132:139–142.
21. Winawer SJ, Zauber AG, O'Brien MJ, Ho MN, Gottlieb L, Sternberg SS, Waye JD, Bond J, Schapiro M, Stewart ET, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps: the National Polyp Study Workgroup. N Engl J Med. 1993; 328:901–906.
22. Triantafyllou K, Papatheodoridis GV, Paspatis GA, Vasilakaki TH, Elemenoglou I, Karamanolis DG. Predictors of the early development of advanced metachronous colon adenomas. Hepatogastroenterology. 1997; 44:533–538.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download