Abstract
Tofacitinib, a novel Janus kinase inhibitor, may prevent structural damage in rheumatoid arthritis (RA). In this cohort study, we compared radiographic progression of hand joints between 21 RA patients who took tofacitinb for 18 months in a phase IIb and its extension study and 42 patients who took conventional disease modifying antirheumatic drugs (DMARDs), using simple erosion narrowing score. For tofacitinib group, changes before and after the treatment were also compared. The changes of erosion and sum scores were significantly less in tofacitinib than DMARDs group (for erosion, -0.60 ± 1.83 vs 0.51 ± 1.77, P = 0.038; for sum, -0.50 ± 1.72 vs 1.57 ± 4.13, P = 0.012). Joint space narrowing score (JSN) was also less in tofacitinib group (0.095 ± 0.58 vs 1.06 ± 2.60, P = 0.055). In tofacitinib group, yearly rates of both erosion and JSN were significantly decreased after administration of tofacitinib (For erosion, 0.62 ± 0.93 to -0.14 ± 0.48, P = 0.009; for JSN, 0.47 ± 0.64 to 0.03 ± 0.40, P = 0.032), as was change of sum score (1.09 ± 1.27 to -0.10 ± 0.63, P < 0.001). In conclusion, tofacitinib may prevent structural damage caused by RA.
Rheumatoid arthritis (RA) is a chronic inflammatory polyarthritis leading to progressive structural damage of joints (1). Joint damage is caused by osteoclasts which are activated by RANKL-RANK interaction (2). Proinflammatory cytokines and CD4+ T lymphocytes can modulate this activation process (2, 3).
Since joint destruction leads to poor health outcome and quality of life (4), it is a major outcome variable in evaluation of RA (5). Severity of joint damage in RA can be objectively measured by counting the numbers of bony erosions and joint space narrowing (JSN) in radiographs of the affected joints (6). Some of the classical disease modifying anti-rheumatic drugs (DMARDs) can partially retard the progression of joint destruction (7, 8), while biologic agents show more protective role in the prevention of joint destruction (2).
Tofacitinib is an orally active janus kinase (JAK) inhibitor which is being applied to various autoimmune diseases which include RA, psoriasis and transplantation (9). Its clinical efficacy in RA was established in a 6-week phase 2A study where objective and subjective clinical endpoints were achieved (10, 11). It inhibits the function of lymphocytes and their inflammatory cytokines by inhibiting signal transduction pathways of JAKs (12). Representative cytokines inhibited include IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 whose receptors have JAK3-mediated common γ chain (12).
Multiple effects of tofacitinib on inflammatory cytokines and T lymphocytes suggest that it can protect joint damage by modulation of osteoclasts. However, objective evidence of its efficacy on structural protection has not yet been reported. Therefore, we reviewed radiographic changes of RA patients who took part in phase 2B clinical trials of tofacitinib from our hospital.
This is a retrospective cohort study comparing radiographic changes of hand joints in patients with RA. The study was composed of 2 parts. In the first part, we compared the radiographic changes of hand joints between 21 patients who took tofacitinib for 12 to 18 months (tofacitinib group) and 42 patients who took conventional DMARDs in the same hospital during the same period (DMARD group). In the second part, radiographic progression rates after the administration of tofacitinib were compared with those before the administration in 17 patients of tofacitinib group, excluding 4 patients in whom less than 90 days had elapsed after the original radiographs had been taken.
Tofacitinib group was composed of 21 patients who had taken part in a 6-month phase IIb tofacitinib monotherapy study and its extension study at Seoul National University Hospital between February, 2008 and December, 2009. The original enrollment criteria for the phase IIb study were active RA patients who showed inadequate response to DMARDs with tender joint count ≥ 6, swollen joint count ≥ 6 and elevation of acute inflammatory markers (ESR > 20 mm/hr and C-reactive protein > 0.7 mg/dL) (13). Among the 21 patients, 20 patients showed inadequate response to DMARD anchoring methotrexate (MTX) during their disease course. Hydroxychloroquine (HCQ) was allowed in the original protocol and 9 patients took the HCQ. In the first 6 months of the phase IIb trial, 2 patients took tofacitinib 1 mg bid, 5 patients 3 mg bid, 4 patients 5 mg bid, 2 patients 10 mg bid, 2 patients 15 mg bid, 2 patients adalimumab and 4 patients placebo, while all patients took tofacitinib 5mg bid for 12 months in the extension study. DMARD group was composed of 42 patients who have never been exposed to tofacitinib and treated with conventional DMARDs at the same hospital during the same period as tofacitinib group. Conventional DMARDs included 35 MTX-, 2 lefluniomide, 2 bucillamine, 1 sufasalzaine-anchored medications and 3 cases of hydroxychloroquine monotherapy. It includes all the patients in whom serial hand X-rays were available during the study period. Enrolled 63 patients fulfilled the 1987 revised American College of Rheumatology criteria for RA (14). The institutional review board of Seoul National University Hospital approved this study.
Hand X-rays were taken for posterior-anterior (PA) and oblique views using Philips Digital Diagnost VM (Philips, Eindhoven, North Brabant, The Netherlands). For the first part of study, radiographs taken at enrollment and 18-months after administration of tofacitinib or DMARDs were compared. For the second part, radiographs were assessed which were taken before the administration of tofacitinib, at the start of tofacitinib and 18 months after the administration. Radiographs were assessed based on the simple erosion and narrowing score (SENS) method (15). Original SENS evaluates radiographic erosion and joint space narrowing in both hands and feet (15). However, only SENS score in hands can be evaluated because only hand X-rays are measured regularly in our clinical practice. Therefore, maximum score was 32 points for erosion, 30 points for joint space narrowing and 62 points for sum. Two rheumatologists measured SENS and their mean scores were used for all the calculations. Each rheumatologist measured SENS with blinded to treatment status of the patients and the other reader' score. However, each reader read the score in sequential order in the second part study. Interobserver correlation coefficients (r) for the two were 0.95 for erosion and 0.90 for JSN.
For the first part, Mann-Whitney test was applied to compare the mean change of erosion, joint space narrowing and sum scores of SENS between tofacitinib and conventional DMARD groups. For the second part, changes of yearly radiographic progression rates were compared before and after the administration of tofacitinib using Wilcoxon matched pairs signed-ranks test. P value < 0.05 was considered to be significant in all of the analyses. All the statistical tests were performed with STATA 9.0 (StataCorp, College Station, TX, USA).
The baseline characteristics of the patients are summarized in Table 1. Tofacitinib and DMARD group showed comparable distributions of age, sex, and positivity of rheumatoid factor and number of DMARDs previously used. However, disease duration was shorter in DMARD group and baseline scores of erosion and JSN were lower in DMARD compared with tofacitinib group.
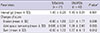
SENS change was significantly lower in tofacitinib than DMARD group. Changes of erosion score after 18 months were significantly less in tofacinitib than in DMARD group (-0.60 ± 1.83 vs 0.51 ± 1.77, P = 0.038), and so was the change of sum score (-0.50 ± 1.72 vs 1.57 ± 4.13, P = 0.012) (Table 2). Difference of SENS changes was still significant after excluding 6 patients who were randomized to adalimumab or placebo for 6-month phase IIb trial (-0.77 ± 1.94 vs 1.57 ± 4.13, P = 0.012) (Table 3).
The mean interval of hand radiographs was 2.98 yr before tofacitinib administration and 1.49 yr after the administration of DMARDs. Both yearly erosion and JSN rates became significantly slowed after administration of tofacitinib. Overall, total rate became favorable after the administration (from 1.09 ± 1.27 per year to -0.10 ± 0.63 per year, P < 0.001) (Table 4).
We showed in this report that tofacitinib can retard radiographic progression of hand joints in DMARD-refractory RA patients compared with conventional DMARD treatment.
In the first part of the study, radiographic erosion rate and total progression rate were significantly lower in tofacitinib group than in conventional DMARD group. Lower rates might come from difference of baseline characteristics of tofacitinib and conventional groups, which is an inherent limitation of retrospective study. However, it is more likely to be a difference caused by treatment effect, considering the following reasons. First, both groups are composed of established RA patients whose disease durations are more than 5 yr. It is known that radiographic progression rate is relatively constant after initial rapid progression (16-18). Second, baseline disease activity of tofacitinib group might be similar to control group considering comparable acute phase reactants in both groups. Tofacitinib group might be more active group since patients of tofacitinib group are refractory to DMARDs while those of control group responded to conventional DMARDs.
In DMARD group, new erosion developed in mean 0.57 joints while new JSN developed in mean 1.19 joints during 18 months. Yearly progression rates in early RA patients were reported to be 4 to 14 units per year when measured by modified Sharp score in which maximum score can be up to 448 (19). The rates of our study seem reasonable, considering that our patient group was an established patients rather than newly-onset RA and only hand joints were evaluated with SENS (maximum score 62) (20).
In the second part of the study, radiographic progression rates were compared before and after the administration of tofacitinib. All of the patients in this group had showed inadequate response to methotrexate, which is considered to be one of the most protective agents against radiographic progression among the conventional DMARDs (21). Flattening of radiographic progression after introduction of tofacitinib suggests that tofacitinib may show more protective effect than any other kind of conventional DMARDs. The second part of study clearly confirms the first part of study by eliminating the confounding effects possibly coming from difference of baseline characteristics.
In this study, we used SENS to measure radiographic damage. For radiographic assessment of RA, Sharp score or its modified forms are commonly used for clinical trial purpose. However, they are too complex to apply to clinical practice, requiring special training. In contrast, SENS is simple and relatively easy to learn (20). It's validity and reliability had been well established (15, 20).
This is a retrospective cohort study and there are several limitations inherent to the study design. First, the data on several risk factors which may affect the structural damage of joints were not available from DMARD group, which included DAS28 score, tender, swollen joint counts at baseline. This was inevitable because joint counts are not routinely measured in our clinical practice. Second, the number of patients may not be enough. We enrolled all the patients who took part in the phase IIb and its extension trials of tofacitinib from our hospital. Despite the small number of patients, significant protective effect was observed. Third, this study was done in Korean patients. Therefore, the favorable result in this study may not be applicable to other ethnic groups. A large-scale randomized prospective study is warranted to confirm our results.
While preparing the manuscript, novel findings were reported on tofacitinib. The clinical efficacy of tofacitinib was confirmed in RA patients who showed inadequate response to MTX or TNF-inhibitors (22-24). One year-structural preventive effect was also shown for tofacitinib in the background of MTX (25). However, structural preventive effect of tofacitinib monotherapy or the lasting effect for more than 1 yr is still unknown, which is proved in this manuscript.
In conclusion, tofacitinib, a novel JAK inhibitor, can prevent structural damage of RA in clinical practice setting.
Figures and Tables
Table 1
Baseline characteristics of the enrolled patients

*The values are mean ± standard deviation. DMARD, disease modifying anti-rheumatic drug; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; DAS-28, disease activity score-28; HAQ-DI, health assessment questionnaire-disability index; SENS, simple erosion and narrowing score; NSAID, non-steroidal anti-inflammatory drug.
Table 2
Radiographic progression between patients who took tofacitinib and those who took conventional DMARDs. SENS score change during the interval was compared between the groups
Notes
References
1. McInnes IB, O'Dell JR. State-of-the-art: rheumatoid arthritis. Ann Rheum Dis. 2010; 69:1898–1906.
2. Karmakar S, Kay J, Gravallese EM. Bone damage in rheumatoid arthritis: mechanistic insights and approaches to prevention. Rheum Dis Clin North Am. 2010; 36:385–404.
3. Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999; 402:304–309.
4. Van der Heijde D, Landewé R, van Vollenhoven R, Fatenejad S, Klareskog L. Level of radiographic damage and radiographic progression are determinants of physical function: a longitudinal analysis of the TEMPO trial. Ann Rheum Dis. 2008; 67:1267–1270.
5. Van der Heijde DM. Radiographic imaging: the 'gold standard' for assessment of disease progression in rheumatoid arthritis. Rheumatology (Oxford). 2000; 39:9–16.
6. Van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 1999; 26:743–745.
7. Ideguchi H, Ohno S, Hattori H, Senuma A, Ishigatsubo Y. Bone erosions in rheumatoid arthritis can be repaired through reduction in disease activity with conventional disease-modifying antirheumatic drugs. Arthritis Res Ther. 2006; 8:R76.
8. Abu-Shakra M, Toker R, Flusser D, Flusser G, Friger M, Sukenik S, Buskila D. Clinical and radiographic outcomes of rheumatoid arthritis patients not treated with disease-modifying drugs. Arthritis Rheum. 1998; 41:1190–1195.
9. Flanagan ME, Blumenkopf TA, Brissette WH, Brown MF, Casavant JM, Shang-Poa C, Doty JL, Elliott EA, Fisher MB, Hines M, et al. Discovery of CP-690,550: a potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J Med Chem. 2010; 53:8468–8484.
10. Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, Krishnaswami S, Burgos-Vargas R, Wilkinson B, Zerbini CA, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009; 60:1895–1905.
11. Coombs JH, Bloom BJ, Breedveld FC, Fletcher MP, Gruben D, Kremer JM, Burgos-Vargas R, Wilkinson B, Zerbini CA, Zwillich SH. Improved pain, physical functioning and health status in patients with rheumatoid arthritis treated with CP-690,550, an orally active Janus kinase (JAK) inhibitor: results from a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2010; 69:413–416.
12. Ju W, Zhang M, Jiang JK, Thomas CJ, Oh U, Bryant BR, Chen J, Sato N, Tagaya Y, Morris JC, et al. CP-690,550, a therapeutic agent, inhibits cytokine-mediated Jak3 activation and proliferation of T cells from patients with ATL and HAM/TSP. Blood. 2011; 117:1938–1946.
13. Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, Connell CA, Gruben D, Krishnaswami S, Wallenstein G, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying anti-rheumatic drugs. Arthritis Rheum. 2012; 64:617–629.
14. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988; 31:315–324.
15. Van der Heijde D, Dankert T, Nieman F, Rau R, Boers M. Reliability and sensitivity to change of a simplification of the Sharp/van der Heijde radiological assessment in rheumatoid arthritis. Rheumatology (Oxford). 1999; 38:941–947.
16. Hulsmans HM, Jacobs JW, van der Heijde DM, van Albada-Kuipers GA, Schenk Y, Bijlsma JW. The course of radiologic damage during the first six years of rheumatoid arthritis. Arthritis Rheum. 2000; 43:1927–1940.
17. Sharp JT, Wolfe F, Mitchell DM, Bloch DA. The progression of erosion and joint space narrowing scores in rheumatoid arthritis during the first twenty-five years of disease. Arthritis Rheum. 1991; 34:660–668.
18. Tiippana-Kinnunen T, Laasonen L, Kautiainen H, Paimela L, Leirisalo-Repo M. Impact of early radiographic remission on the 15-year radiographic outcome in patients with rheumatoid arthritis. Scand J Rheumatol. 2011; 40:263–268.
19. Van der Heijde DM, van Leeuwen MA, van Riel PL, Koster AM, van't Hof AM, van Rijswijk MH, van de Putte LB. Biannual radiographic assessments of hands and feet in a three-year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum. 1992; 35:26–34.
20. Dias EM, Lukas C, Landewé R, Fatenejad S, van der Heijde D. Reliability and sensitivity to change of the Simple Erosion Narrowing Score compared with the Sharp-van der Heijde method for scoring radiographs in rheumatoid arthritis. Ann Rheum Dis. 2008; 67:375–379.
21. Pincus T, Ferraccioli G, Sokka T, Larsen A, Rau R, Kushner I, Wolfe F. Evidence from clinical trials and long-term observational studies that disease-modifying anti-rheumatic drugs slow radiographic progression in rheumatoid arthritis: updating a 1983 review. Rheumatology (Oxford). 2002; 41:1346–1356.
22. Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, Gruben D, Wallenstein GV, Zwillich SH, Kanik KS. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012; 367:495–507.
23. Van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, Forejtova S, Zwillich SH, Gruben D, Koncz T, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012; 367:508–519.
24. Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, Gruben D, Wallenstein G, Krishnaswami S, Zwillich SH, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013; 381:451–460.
25. Van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, Cardiel MH, Cohen S, Nash P, Song YW, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013; 65:559–570.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download