Abstract
To identify a prognostic marker that is less sensitive to variations in the elapsed time since paraquat ingestion, we assessed the time between paraquat ingestion and a negative dithionite urine test as a prognostic parameter in patients with acute paraquat intoxication. Forty-one patients with acute paraquat intoxication were enrolled in this study and analyzed to verify significant determinants of mortality and organ dysfunction. The amount of paraquat ingested, paraquat plasma levels, and the time to a negative urine dithionite test were significant independent risk factors predicting mortality. The amount of paraquat ingestion, and the time to a negative urine dithionite test were independent risk factors predicting organ dysfunction. With a cut-off value of 34.5 hr for the time to negative conversion of the urine dithionite test, the sensitivity and specificity for mortality were 71.4% and 75.0%, respectively. The incidence of acute kidney injury and respiratory failure above 34.5 hr were 100% and 85.0%, respectively. In conclusion, the time to a negative urine dithionite test is the reliable marker for predicting mortality and/or essential organ failure in patients with acute paraquat intoxication, who survive 72 hr.
Intentional or accidental ingestion of Paraquat (PQ; 1,1-dimethyl-4-4-bipyridium dichloride) is frequently fatal as a result of multiple organ failure. Over the past 30 yr, several methods have been studied for modifying the toxicity of PQ (1-5), but these have not proved effective and thus, the clinical outcome is usually determined by the degree of exposure to PQ.
Due to the high mortality associated with PQ poisoning, several parameters have been proposed as prognostic indicators: plasma and urinary PQ concentrations, respiratory index (6), serum creatinine and potassium (7), and arterial blood bicarbonate and base excess levels (8). We have previously reported that initial routine laboratory parameters, including arterial blood gas analysis, renal function, and liver function, are good prognostic markers in acute PQ intoxication. Among these, the plasma PQ level at a given time has been regarded as the most reliable parameter for prediction of the clinical outcome (9). Patients whose plasma PQ levels are below 2.0, 0.6, 0.3, 0.16, and 0.1 mg/L at 4, 6, 10, 16, and 24 hr, respectively, are likely to survive (10). However, even by using these numerical predictive levels, it is difficult to determine which patients will survive in a clinical setting, because some patients with low PQ levels nevertheless die. Recently, we reported that mortality occurs at such low plasma PQ concentrations (11).
The primary cause of death is cardiac arrest during the first 2-3 days. After then, respiratory failure is the primary cause of death (12, 13). Renal failure is also a critical complication (14). Therefore, prognostic markers predicting these organ failures are required for use in a clinical setting.
There are various possible explanations for why the plasma level of PQ is a less reliable clinical marker than expected. First, the plasma PQ level does not necessarily represent the ingested amount or the body burden of PQ, particularly when measured during the first few hours. The plasma level peaks early, 1 hr after PQ ingestion, followed by a rapid decline with a steep gradient due to rapid distribution from circulation to other compartments. During this period, the plasma concentration has substantial variations in concentration even with slight changes in the time interval since ingestion (Fig. 1). Second, patients arrive at various lengths of time after PQ ingestion. It can be difficult to determine how long it has been elapsed since PQ ingestion because patients may not remember the exact time of ingestion and may give general descriptions such as "after breakfast" or "around 9:00."
Considering that PQ intoxication has a dose-response relationship, it is appropriate to evaluate potentially reliable indicators of the PQ dose other than the plasma PQ level at a given time. The aim of this study was to seek a prognostic marker that is less influenced by the time elapsed since PQ ingestion and the plasma PQ level in patients with acute PQ intoxication.
We enrolled 95 patients (65 males and 30 females, aged 54.8 ± 15.7 yr) with acute PQ intoxication in this study (Fig. 2). All of the patients ingested concentrated PQ (22%-23% per volume) while attempting suicide and were admitted to the Institute of Pesticide Poisoning, Soonchunhyang University Cheonan Hospital from May to September 2011. For these patients, the dithionite urine PQ test showed strong positive at emergency room (ER). Exclusion criteria included weak or negative dithionite urine test at the ER and/or the amount of PQ ingested was uncertain. In addition, in our clinical experience with thousands of cases with acute PQ intoxication, it takes time for organ failure to develop. If the patient dies early, organ failure would not be detected. Therefore, any patients who died within 72 hr of admission were excluded from the evaluation of organ failure. The amount ingested was estimated from the number of swallows, where one mouthful was considered 20 mL.
Upon admission, all patients received the following standardized medical emergency treatment. In brief, a gastric lavage was performed on all subjects who presented to the ER within 2 hr of PQ ingestion. For those whose intoxication occurred within 12 hr before presenting to the ER, 100 g of Fuller's earth in 200 mL 20% mannitol was administered. Hemoperfusion (Absorba 300; Gambro, Hechingen, Germany) was performed for 3.5 hr on the results of the urine dithionite test. Hemoperfusion was performed for up to 7 hr with cartilage exchange in several paraquat intoxication cases which the urine dithionite test was positive after first hemoperfusion. Blood flow was 200-250 mL, and heparin was used as the primary anticoagulant. Catheters were placed upon admission to the ER; jugular catheters were preferred, and femoral or subclavian catheters were placed as a second choice. Glutathione (50 mg/kg • 24 hr) was administered intravenously for 3 additional days as a reactive oxygen species scavenger (4).
Blood samples for a complete blood count and blood chemistry were collected at the ER and every day for the first 3 hospital days, followed by twice a week. Arterial blood gas analysis was measured at the ER and every hospital day in the morning. Plasma PQ levels were measured at the ER using high-performance liquid chromatography.
Ten milliliters of urine was placed into a beaker, 2 g of sodium bicarbonate was added, and the mixture was shaken gently. One gram of sodium dithionite was added, the effervescence was allowed to subside, and the mixture was shaken again. The solid material was allowed to settle and the mixture was viewed against a white background. The results are presented as grades 1-4: black (+4), deep blue (+3), light blue (+2), and barely distinguishable blue (+1). In our preliminary study, the cut-off value for PQ detection (+1) was 1 µg/mL.
When the patients arrived at the ER, a Foley catheter was inserted and all of the urine in the bladder was collected in a container. Urine collected immediately afterward was placed in another container and dithionite urine tests were conducted on these two urine samples. The first urine sample represented the urine produced over the previous several hours. Therefore, the results of this dithionite test represented the average blood PQ levels during the previous several hours. The dithionite test in the second urine sample represented the current blood PQ level. If the PQ level in the first urine sample was higher than in the second urine sample, the blood PQ level would have been higher in the previous several hours than at the ER and vice versa.
After the second dithionite urine test, sequential dithionite urine tests were conducted every 3-4 hr until the results became trace (light blue color). From this point on, the dithionite urine test was conducted every hour until the blue color disappeared. Patients who died before the urine dithionite test became negative were excluded from this study. The time interval from PQ ingestion to a negative dithionite urine test was calculated.
Acute kidney injury (AKI) was defined as an initial serum creatinine level of > 1.2 mg/dL, or a ≥ 50% increase in serum creatinine from baseline, based on the RIFLE criteria (15). Respiratory failure was defined as hypoxia (PaO2 < 60 mmHg or a decrease of ≥ 30% from basal PaO2 levels). Hepatic dysfunction was defined as the greater of AST or ALT ≥ 80 U/L or more than twice the normal cut-off level.
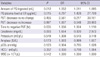
Continuous variables are presented as means ± standard deviations and categorical variables as frequency (in percent). Differences between the survival and mortality groups were compared using Mann-Whitney-U test for continuous variables and using the chi-squared or Fisher's exact test for categorical variables (Table 1).
Multivariate logistic regression analysis was used to identify significant determinants of mortality (Table 2) and organ dysfunction (Table 3). Variables were adjusted by age, gender, and the time elapsed between PQ ingestion and hospital arrival. The results were reported as odds ratios (ORs) with 95% confidence intervals (CIs).
To develop a cut-off point for time to negative conversion of the urine dithionite test, receiver operating characteristic (ROC) curve analysis was used to provide the optimal sensitivity and specificity for mortality. Statistical analyses were performed using SPSS software (version 14.0; SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Demographic characteristics and baseline laboratory results for the survival and mortality groups of patients are summarized in Table 1. The amount of PQ ingested, plasma PQ levels at the ER, and the results of the urine dithionite tests were significantly higher in the mortality group. Serum creatinine level was significantly higher in the mortality group. Twenty-one (51.2%) patients died during hospitalization. The causes of death were acute respiratory failure for 21 patients (100.0%).
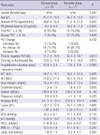
Multivariate binary logistic regression analysis was conducted to verify significant determinants of mortality. The amount of PQ ingested, PQ levels at the ER, and the time to a negative urine dithionite test were independent risk factors predicting mortality (Table 2).
Multivariate binary logistic regression analysis was conducted to verify significant determinants of organ dysfunction. Of the 41 patients, acute kidney injury occurred in 35 patients (85.4%), acute respiratory failure in 26 patients (63.4%), and hepatic dysfunction in 19 patients (46.3%), in that order. With each additional 1 mL ingested, the patient's risk of respiratory failure increased by 6%. Moreover, the longer the time to negative conversion of the urine dithionite test, the higher the risk of acute kidney injury (by 25%) and respiratory failure (by 6%) (Table 3).
With a cut-off value of 30.5 hr to a negative urine dithionate test, the sensitivity and specificity for identifying patients in the mortality group were 81.0% and 60.0%, respectively (AUC, 0.725). Similarly, the sensitivity and specificity for identifying patients in the mortality group were 71.4% and 75.0% with a cut-off value of 34.5 hr. Using these data, we selected a time to negative conversion of the urine dithionite test of 34.5 hr as a cut-off value. Time to a negative dithionite urine test, mortality rate, the incidence of acute kidney injury, and acute respiratory failure were significantly higher in the "above 34.5 hr patient group" than in the "below 34.5 hr patient group" (Table 4).
The principal result of the present study is that the time between PQ ingestion and a negative dithionite urine test is an independent risk factor predicting not only death but also organ failure, particularly for the lungs and kidneys. Therefore, it is important to understand the mechanism of the dithionite urine test.
A variety of spectrophotometric (16, 17), gas and liquid chromatographic (18, 19), and radioimmunoassay techniques (20, 21) have been applied to measuring PQ in biological fluids. However, the majority of these methods cannot provide an accurate and reliable quantitative result in an emergency situation; thus, further development of suitable rapid techniques is desirable. Sodium dithionite is the sodium salt of dithionous acid. It is often used in physiology experiments to lower the redox potential of a solution (22). PQ is determined in a dilute solution by measuring the optical density of the blue-colored PQ free radical produced by reduction by alkaline sodium dithionite. However, when the intensity of the blue color is measured with the naked eye, the detection limit for PQ is ~1 µg/mL. The advantages and disadvantages of the dithionate test are that it is an easy and simple test producing rapid results but with low sensitivity. Thus, a negative dithionite test does not mean that no PQ is present in the urine. Using a more sensitive method (e.g. HPLC), PQ should be detectable for a longer period of time after ingestion at concentrations below 1 µg/mL.
To understand the meaning of the time to a negative dithionite urine test, therefore, we review the toxicokinetics of PQ. The distribution volume of PQ has been reported as 1.2-1.6 L/kg after oral ingestion in humans (23). A 3-compartment model best describes the subsequent distribution of PQ: plasma, a rapid uptake and removal compartment (the kidneys), and a slow uptake compartment peaking at about 4-5 hr (the lungs). Plasma levels reach a peak early, 1 hr after PQ ingestion, followed by a rapid decline with a steep gradient and then a gentle slope over a long elimination period. The long elimination half-life (tβ) has been estimated as 84 hr (24). PQ is not metabolized or biotransformed in the human body and is primarily eliminated through the kidneys. Based on this, we believe that the time to a negative dithionite urine test is a function of the total PQ in all of the compartments and of renal function.
Early deterioration of renal function is a critical complication in acute PQ intoxication. Fortunately, renal excretion of PQ is excellent and most PQ is eliminated through the kidneys as long as renal function remains normal (24). Kidney injury develops in a plasma PQ concentration-dependant manner and results in delayed excretion, further exacerbating the potential for injury. Recently, we reported clinical features of acute kidney injury, estimated based on serum creatinine clearance in patients with acute PQ intoxication (14). The peak of serum creatinine was present on the fifth day after PQ ingestion and normalized within 3 wk. We also reported that the early kidney injury markers neutrophil gelatinase-associated lipocalin and kidney injury molecule 1 increased 24-48 hr after PQ ingestion (16). Thus, the time at which the PQ level in the urine will become undetectable depends on the degree of renal injury and the PQ burden in the body. In addition to the time to a negative urine dithionite test, the amount of PQ ingested and PQ levels at the ER were significant independent risk factors predicting mortality (Table 2).
The amount of PQ ingested, and the time to a negative urine dithionite test were independent risk factors predicting organ failure. The PQ level at the ER might not predict any organ injury, although the 95% confidence interval widely ranged. Therefore, if we could ascertain the precise amount of PQ ingested and the time to a negative urine dithionite test, it would be more prognostic supported by both factors, predicting both mortality and organ failure. In our study, the amount of PQ ingested was estimated based on the number of swallows. To determine the volume of a mouthful, we measured mouthfuls in adults; the average was 20 mL, similar to previous reports (13-15). However, in some instances, we could not confirm the precise amount of PQ ingested because the patient stated that they drank a cup, a bowl, or a glass. Others did not remember the exact time or amount because they were upset and/or drunken when they ingested the PQ. The exact time interval is important in interpreting the results of the negative dithionite urine test. However, it is not as critical as in the interpretation of the plasma PQ level, because the latter has greater variations in concentration even with slight variations in the time interval after PQ ingestion.
In conclusion, the time to a negative urine dithionite test is the reliable marker for predicting mortality and/or essential organ failure in patients with acute PQ intoxication, who survive after 72 hr.
Figures and Tables
Fig. 1
Plasma paraquat levels of two patients who ingested paraquat. The case-1, 48-yr-old man, arrived at hospital 5 hr after 200 mL of paraquat ingestion and the initial plasma paraquat level was 29.8 µg/mL. He discharged moribundly to other hospital in the second hospital day. The case-2, 58-yr-old man, arrived 6 hr after 50 mL of paraquat ingestion and the initial plasma paraquat level was 4.2 µg/mL. He died 72 hr after paraquat intoxication because of cardiac arrest and acute respiratory failure. The plasma paraquat levels of both patients demonstrated transient increases within 7 hr of paraquat ingestion followed by an abrupt drop. The plasma concentrations demonstrate substantial variations in concentration even with slight variations in the time interval since ingestion.

Table 2
Multivariate binary logistic regression analysis to identify significant determinants of mortality
References
1. Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011. 72:745–757.
2. Lin JL, Lin-Tan DT, Chen KH, Huang WH, Hsu CW, Hsu HH, Yen TH. Improved survival in severe paraquat poisoning with repeated pulse therapy of cyclophosphamide and steroids. Intensive Care Med. 2011. 37:1006–1013.
3. Moon JM, Chun BJ. The efficacy of high doses of vitamin C in patients with paraquat poisoning. Hum Exp Toxicol. 2011. 30:844–850.
4. Kim JH, Gil HW, Yang JO, Lee EY, Hong SY. Effect of glutathione administration on serum levels of reactive oxygen metabolites in patients with paraquat intoxication: a pilot study. Korean J Intern Med. 2010. 25:282–287.
5. Gil HW, Kim SJ, Yang JO, Lee EY, Hong SY. Clinical outcome of hemoperfusion in poisoned patients. Blood Purif. 2010. 30:84–88.
6. Suzuki K, Takasu N, Arita S, Maenosono A, Ishimatsu S, Nishina M, Tanaka S, Kohama A. A new method for predicting the outcome and survival period in paraquat poisoning. Hum Toxicol. 1989. 8:33–38.
7. Ragoucy-Sengler C, Pileire B. A biological index to predict patient outcome in paraquat poisoning. Hum Exp Toxicol. 1996. 15:265–268.
8. Yamaguchi H, Sato S, Watanabe S, Naito H. Pre-embarkment prognostication for acute paraquat poisoning. Hum Exp Toxicol. 1990. 9:381–384.
9. Ikebuchi J, Proudfoot AT, Matsubara K, Hampson EC, Tomita M, Suzuki K, Fuke C, Ijiri I, Tsunerari T, Yuasa I. Toxicological index of paraquat: a new strategy for assessment of severity of paraquat poisoning in 128 patients. Forensic Sci Int. 1993. 59:85–87.
10. Proudfoot AT, Stewart MS, Levitt T, Widdop B. Significance of plasma-paraquat concentrations. Lancet. 1979. 2:330–332.
11. Gil HW, Kang MS, Yang JO, Lee EY, Hong SY. Association between plasma paraquat level and outcome of paraquat poisoning in 375 paraquat poisoning patients. Clin Toxicol (Phila). 2008. 46:515–518.
12. Lee KH, Gil HW, Kim YT, Yang JO, Lee EY, Hong SY. Marked recovery from paraquat-induced lung injury during long-term follow-up. Korean J Intern Med. 2009. 24:95–100.
13. Kim YT, Jou SS, Lee HS, Gil HW, Yang JO, Lee EY, Hong SY. The area of ground glass opacities of the lungs as a predictive factor in acute paraquat intoxication. J Korean Med Sci. 2009. 24:636–640.
14. Kim SJ, Gil HW, Yang JO, Lee EY, Hong SY. The clinical features of acute kidney injury in patients with acute paraquat intoxication. Nephrol Dial Transplant. 2009. 24:1226–1232.
15. Gil HW, Yang JO, Lee EY, Hong SY. Clinical implication of urinary neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 in patients with acute paraquat intoxication. Clin Toxicol (Phila). 2009. 47:870–875.
16. Shivhare P, Gupta VK. Spectrophotometric method for the determination of paraquat in water, grain and plant materials. Analyst. 1991. 116:391–393.
17. de Almeida RM, Yonamine M. Enzymatic-spectrophotometric determination of paraquat in urine samples: a method based on its toxic mechanism. Toxicol Mech Methods. 2010. 20:424–427.
18. Braithwaite RA. Emergency analysis of paraquat in biological fluids. Hum Toxicol. 1987. 6:83–86.
19. Kawase S, Kanno S, Skai S. Determination of the herbicides paraquat and diquat in blood and urine by gas chromatography. J Chromatogr. 1984. 283:231–240.
20. Bowles MR, Eyles DW, Hampson EC, Pond SM. Quantitation of paraquat in biological samples by radioimmunoassay using a monoclonal antibody. Fundam Appl Toxicol. 1992. 19:375–379.
21. Nagao M, Takatori T, Terazawa K, Wu B, Wakasugi C, Masui M, Ikeda H. Development and application of immunoassay for paraquat: radioimmunoassay. J Forensic Sci. 1989. 34:547–552.
22. Mayhew SG. The redox potential of dithionite and SO-2 from equilibrium reactions with flavodoxins, methyl viologen and hydrogen plus hydrogenase. Eur J Biochem. 1978. 85:535–547.
23. Houzé P, Baud FJ, Mouy R, Bismuth C, Bourdon R, Scherrmann JM. Toxicokinetics of paraquat in humans. Hum Exp Toxicol. 1990. 9:5–12.
24. Kang MS, Gil HW, Yang JO, Lee EY, Hong SY. Comparison between kidney and hemoperfusion for paraquat elimination. J Korean Med Sci. 2009. 24:S156–S160.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download