Abstract
Little is known about the prevalence and seroprevalence of low-risk human papillomavirus (HPV) and the risk factors for HPV infection in Korean women. We determined the prevalence of low-risk HPV among 902 women aged 20-59 yr and the seroprevalence of low-risk HPV subtypes 6 and 11 among 1,094 women aged 9-59 yr in the general population. Genital low-risk HPV DNA was assessed by liquid hybridization and polymerase chain reaction. Antibody titers against HPV 6 and 11 were measured by a multiplexed competitive luminex technique. The prevalence of genital low-risk HPV was 4.9%. It reached its highest peak of 10.3% at 20-29 yr of age and a second peak of 3.2% at 50-59 yr of age. The seroprevalence of HPV 6 or 11 was 9.4%. It reached its highest peak of 12.7% at 25-29 yr of age and a second peak of 12.3% at 50-59 yr of age. In multivariable analysis, the number of lifetime sexual partners and past history of sexually transmitted diseases were associated with the seroprevalence but not prevalence of HPV. It is suggested that younger women should receive prophylactic HPV vaccination before they become sexually active and exposed to HPV in their 20s. This study provides baseline data for developing HPV vaccination programs and monitoring vaccine efficacy in Korea.
Human papillomavirus (HPV) is the most common sexually transmitted infection. In one study, approximately half of the women without HPV infection became infected with HPV within 3 yr after initiating sexual activity (1). Currently, more than 100 different HPV subtypes have been identified, which mostly infect genital epithelial cells. HPV subtypes are divided broadly into two groups according to their epidemiological association with cervical cancer. The low-risk HPV group, including HPV subtypes 6 and 11, is associated with benign lesions, genital warts or recurrent respiratory papillomatosis (2). The high-risk HPV group, including HPV subtypes 16 and 18, induces precancerous lesions such as cervical intraepithelial neoplasia (CIN), cervical cancer or ano-genital cancer (3, 4).
After sexual contact, genital warts may occur in the uterine cervix, vagina, vulva, anus and oral cavity. Approximately 90% of genital warts are related to low-risk HPV types 6 and 11 (5-7). The incidence of genital warts has gradually increased since the 1950s (6), and has become a major cause of nononcogenic HPV-related morbidity. In addition, low grade squamous intraepithelial lesions are associated with not only high-risk HPV infection but also low-risk HPV 6 and 11 infection.
Our current epidemiological knowledge of HPV infection is based on the genital HPV DNA test which only identifies the current HPV infection (8). In addition, the HPV DNA test has been applied limitedly because of the difficulty of collecting samples from males and the reluctance to gynecologic examination among females (9-11). In contrast, serum antibody to HPV is a useful, although not perfect, complementary marker reflecting cumulative HPV exposure (8, 12, 13).
In the general population of Korea, very little data exist on the epidemiology of low-risk HPV 6 and 11, which are two of the four types targeted by the quadrivalent HPV vaccine (10, 14, 15). We estimated the prevalence and seroprevalence of low-risk HPV infection in Korean women in order to help establish vaccine policy specific to the characteristics of Korean women and assess vaccine efficacy.
We conducted a survey of the prevalence and seroprevalence of low-risk HPV in Korean women between July 2008 and May 2009. A total of 1,143 women, 9-59 yr of age, who visited our institutions for a regular medical check-up were eligible for this study. All study samples were obtained using a complex, stratified, multistage probability cluster design in order to select a nationally representative sample. For females from 9 to 19 yr of age, the low-risk HPV serologic test was performed without a questionnaire study or a genital HPV DNA test in light of the relatively late age of sexual debut and conservative sexual culture found in Korea. Females 20-59 yr of age were asked to fill out a self-reporting questionnaire containing questions relating to socio-demographic characteristics and lifestyle habits associated with HPV infection, such as sexual behavior, history of sexually transmitted disease (STD), usage of tobacco and usage of oral contraceptives.
Of a total of 1,143 women, the prevalence of low-risk HPV was analyzed in a total of 902 women aged 20-59 yr. This excludes 241 women; one who did not agree to the study, four who did not respond to the questionnaire, eight whose results regarding Hybrid Capture II (HC II) were missing and 228 who were 9-19 yr of age. Of the 1,130 women 9-59 yr of age, comprised of the 228 who were 9-19 yr of age along with the 902 who were 20-59 yr of age, an analysis was performed for the seroprevalence of low-risk HPV in a total of 1,094 females. This excludes 36 women 20 yr of age or older from whom serum samples could not be collected.
Cervicovaginal cells were collected from 902 women 20-59 yr of age using a Cytobrush (Cervibrush, Cell-Path, Herte, United Kingdom). These cells were collected in an Eppendrof tube that contained 20 mL of phosphate buffer solution (PBS). Blood samples of 10 mL were collected from each of 1,094 women 9-59 yr of age using the vacutainer system. Blood samples were centrifuged at 1,500 g for 10 minutes and the serum was separated from the cells. All samples were transported to a central laboratory and stored at -70℃ until processing.
To confirm low-risk HPV group (consisting of HPV subtypes 6, 11, 42, 43, 44) infection and viral load, cervicovaginal cells were harvested using a dacron swab, added to the collection kit (Digene, Gaithersburg, MD, USA) and stored at -20℃ until analysis. RNA probes of low-risk HPV were hybridized with denatured, single-stranded DNA. This reaction mixture was transferred to a tube coated with anti-DNA-RNA hybrid antibodies. The immobilized hybrids were then incubated with an alkaline phosphatase-conjugated antihybrid monoclonal antibody. After rinsing, these reactants were treated with Lumi-Phospho 530, which reacts to alkaline phosphatase, a dioxetane-based chemiluminescent substrate. The light generated by the reaction was measured by luminometer and the intensity was recorded using units which were relative to the reaction. The relative unit for the light intensity was defined as the degree of illumination relative to the positive control group. A positive cutoff value was set at 1 pg HPV DNA per milliliter in each specimen.
For typing of low-risk HPV subtypes 6 and 11, DNA was extracted using the Qiagen DNA extraction kit (Qiagen, Valencia, CA, USA). β-globin PCR was performed to check the DNA quality. GP5+/6+ primer was used on all of the β-globin PCR positive samples to confirm whether they were positive for low-risk HPV subtypes 6 and 11. Samples testing positive for low-risk HPV were examined by PCR using HPV 6 and 11 primer to determine the specific subtype.
The antibodies to low-risk HPV subtypes 6 and 11 were measured by Merck and Co. Inc., using a multiplexed competitive Luminex® assay. The antibody titration method was measured according to the HPV antibody measurement method reported by Opalka et al. in 2003 (16), and reported in milli-Merck units per mL. The monoclonal antibodies used in the HPV multiplexed competitive Luminex assay included H6.M48 and K11. B2 for low-risk HPV subtypes 6 and 11, respectively. Cutoff values for HPV seropositivity were at least 20 and at least 16 mMU/mL for HPV subtypes 6 and 11, respectively (17).
Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated using unconditional multiple logistic regression to assess the associations between HPV DNA/seropositivity and demographic and behavioral characteristics. For all analyses, a significance level of P < 0.05 was chosen.
Table 1 shows the overall and age-specific prevalence of low-risk HPV in the study population. Of the 902 Korean women 20-59 yr of age that were tested for prevalence of HPV, 4.9% (44 of 902) were shown to be positive for low-risk HPV DNA. The age-standardized prevalence, calculated by applying the female population given by the National Statistics Office for Korea in 2008, was shown to be 4.9% (95% CI 2.3-7.6). The prevalence of low-risk HPV reached its highest peak of 10.3% (95% CI 6.2-14.5) among females 20-29 yr of age and decreased thereafter. It then reached a second peak of 3.2% (95% CI 0.9-5.6) among females 50-59 yr of age.
Of the 44 HC II positive samples, only 8 (18.2%) had subtypes confirmed by PCR; the subtypes of the remaining 36 samples were not confirmed. Of the 8 samples whose subtypes were confirmed, 8 samples (18.2%) were positive for HPV 6 and 2 samples (4.5%) were additionally positive for HPV 11, meaning there was simultaneous co-infection with HPV 6. The type-specific prevalence for HPV 6 and HPV 11 were 0.9% and 0.2%, respectively (data not shown).
Of 1,094 females 9-59 yr of age tested for seroprevalence of HPV, the seroprevalence of either low-risk HPV subtypes 6 or 11 was 9.4% (95% CI 7.7-11.3), and the age-standardized seroprevalence was shown to be 9.6% (95% CI 7.8-11.4). The age-specific seroprevalence of low-risk HPV reached its highest peak of 12.7% (95% CI 7.7-19.3) among females 25-29 yr of age. It decreased thereafter but reached a second peak of 12.3% (95% CI 8.2-17.6) among females 50 to 59 yr of age (Fig. 1). The type-specific seroprevalences of low-risk HPV are shown in Table 2. HPV 6 seroprevalence was 8.1% (95% CI 6.6-9.9), and HPV 11 seroprevalence was 3.9% (95% CI 2.9-5.3). Peak seroprevalence occurred in the 30-39 yr-old age group for HPV 6 (11.4%, 95% CI 7.5-16.3) and in the 25-29 yr-old age group for HPV 11 (7.0%, 95% CI 3.4-12.6). Just like the overall seroprevalence of low-risk HPV, the type-specific seroprevalences for HPV 6 and 11 reached second peaks in the 50-59 yr-old age group. Seropositivity for both low-risk HPV 6 and 11 was rare (2.6%, 95% CI 1.8-3.8) and reached its highest peak among females aged 25-29 yr.
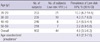
Table 3 presents the concordance between low-risk HPV DNA positivity and seropositivity. A total of 868 women aged 20-59 yr were tested with both HC II for the presence of low-risk HPV DNA and a HPV serologic test for seropositivity of low-risk HPV subtypes 6 and 11. Of the 44 HC II positive samples, only 8 had subtypes confirmed by PCR. Among females who were PCR-positive for HPV 6 and 11, the proportions who were also seropositive to the respective type were 1/8 (12.5%) and 0/2 (0%), respectively. Among PCR-negative for HPV 6 and 11, the proportions that were seropositive to the respective type were 83/860 (9.7%) and 39/866 (4.5%), respectively.
We analyzed the relationship between socio-demographic and behavioral characteristics and the seroprevalence of low-risk HPV. Seroprevalence of low-risk HPV was significantly associated with the lifetime number of sexual partners and past history of STD. The seroprevalence of low-risk HPV increased from 8.7% among females with 1 lifetime sexual partner to 17.6% among those with 3 lifetime sexual partners (P = 0.028). We also found that past history of STD was significantly associated with the seroprevalence of low-risk HPV (P = 0.001). The effects of the remaining risk factors were not statistically significant (Table 4).
This is the first study to describe the seroprevalence along with the prevalence of low-risk HPV in Korean women. The prevalence of genital low-risk HPV infection detected by HC II was shown to be 4.9% (the age-standardized prevalence was 4.9%). This is comparable to a low-risk HPV prevalence of 4.1% reported by Shin et al. in 2003 (15) using the PCR method and lower than that reported in the United States (18). Low-risk HPV prevalence in men was 0%-43% in several studies and 2.6% in Korean university students (10). In regard to the age-specific prevalence of low-risk HPV, it reached its highest peak at 20-29 yr of age, and afterward, it showed a tendency to decrease, but reaching a second peak at 50-59 yr of age. This is similar to previously reported results (19). The age-specific pattern we found can be explained by multiple factors. One possible explanation is that middle-aged women have new sexual partners as they become more sexually active, and thus the possibility of new HPV infection becomes high. Another is the reactivation of latent HPV infection due to the deterioration of immunity as a result of hormonal deficiency during the perimenopausal period. Host factor such as immune-compromised status can play a role in the HPV infection (20). A third explanation is the cohort effect: prevalence appears to be high, but in reality patients were unaware of an infection that occurred in their 20s which was detected only from the results of tests performed later (19, 21, 22).
Concerning the distribution of low-risk HPV subtypes, among 44 HC II positive samples, HPV 6 was detected in 8 cases and HPV 11 was detected in 2 cases. 81.8% (36/44) of the HC II positive samples had subtypes that could not be determined. This may be due to that the PCR primers used in our study were only for HPV subtypes 6 and 11, and thus the remaining subtypes could not be detected.
Because the clearance rate for low-risk HPV was higher than that for high-risk HPV, with a shorter duration of infection, the prevalence of low-risk HPV might reflect a pattern of new HPV infections (23). On the other hand, HPV seroprevalence is a good marker of cumulative HPV exposure, although due to a low seroconversion rate after HPV infection, HPV seroprevalence does not indicate the current state of HPV infection (8, 12). Worldwide, the seroprevalence of low-risk HPV varies according to HPV subtype, age, and regional differences. Nonetheless, it has been reported to be 10%-20% (24, 25). The seroprevalence of low-risk HPV subtypes 6 and 11 was 9.4% among females aged 9-59 yr (the age-standardized prevalence was 9.6%), which was lower than those of western countries. In the present study, the seroprevalence of low-risk HPV subtypes 6 and 11 according to age showed its highest peak in the 20s and a second peak in the 50s, similar to the age-specific pattern of the prevalence of HPV infection. It was observed in previous reports that generally, as age increased, HPV seroprevalence also increased or maintained a plateau level (25, 26). Additionally, it has been reported that HPV prevalence reaches a peak within 5-10 yr of sexual debut and that HPV seroconversion occurs frequently between 6 and 18 months of DNA detection (12, 27, 28). Therefore, we can infer that first exposure to HPV among Korean women is occurring earlier, and that the sexual lives of middle-aged women have become more active.
The concordance between low-risk HPV DNA positivity and seropositivity was poor. Only 12.5% of HPV 6 PCR-positive women were seropositive for HPV 6. In case of HPV 11, two PCR-positive samples had no antibody for HPV 11. This discrepancy can be explained by the effect of HPV-specific immune-evasion mechanism, waning of detectable antibodies, a long lag time required for seroconversion (29, 30).
In regard to behavioral risk factors, there were significant differences in seroprevalence of low-risk HPV by both lifetime number of sexual partners and history of STD. It had previously been reported that HPV seroprevalence is associated with the age of sexual debut, the lifetime number of sex partners, marital status, and education level (25, 31).
Although clinical trials has not demonstrated remarkable efficacy in prevention of invasive cervical cancer from HPV vaccination, it has shown that the vaccine is almost 100% effective in preventing infection related to the vaccine-specific HPV genotypes. According to previous studies, teenagers without sexual experience certainly benefit from HPV vaccination. Kim et al. reported that HPV vaccine is highly immunogenic and well tolerated in Korean girls aged 10-14 yr (32). Among sexually active women, cases where individuals were infected with all four types of HPV vaccine were rare. Therefore, even women infected with one or more HPV subtypes prior to vaccination can be protected from uninfected subtypes by vaccination. Considering that as women become older or more sexually active, the possibility of more sexual partners and the exposure to HPV increases, most women could benefit from HPV vaccination.
There are limitations in our study. First, although the low-risk HPV group is often associated with low grade squamous intraepithelial lesions and non-malignant genital tract lesions (genital warts), such correlation between the low-risk HPV positivity and cervical pathology was not analyzed in our study. Thereby, a further study is needed to monitor the presence and persistence of cervical pathology in low-risk HPV infected women. Second, in our study, PCR assay was performed to classify low-risk HPV subtypes 6 and 11 among various HC II positive samples. It is possible that some false negative samples of HC II were missed while other low risk HPV types except HPV 6 and 11 could not have been detected. For these reasons, we may be underestimating the low-risk HPV prevalence in our study.
In conclusion, our results confirm that the prevalence and seroprevalence of low-risk HPV tend to increase rapidly in their 20s, when women are becoming sexually active. In order to achieve optimal efficacy, it would be reasonable to vaccinate women in their teens prior to HPV exposure.
However, our study shows that seroprevalence reaches its peak earlier than it has been previously thought, implying that the pattern of sexual behavior among Korean teenagers is changing rapidly. Therefore, also considering the lag period of seroconversion, the optimal age for catch-up vaccination should be lowered. In addition, our results show that a second peak of both the prevalence and seroprevalence of low-risk HPV occurs in middle aged women. Also considering that the probability of women who are sexually active being infected with all four HPV vaccine types is low, it may be necessary to adjust the recommended age of catch-up vaccination and discuss the vaccination strategy in middle aged woman. However, future research will be needed to define the clinical significance and natural history of seronegative infection of low-risk HPV. Our data will help to establish a policy to reduce an important fraction of disease burden of low-risk HPV 6 and 11 targeted by the quadrivalent vaccine and to monitor vaccine efficacy.
Figures and Tables
Table 3
Concordance between genital low-risk HPV 6 and 11 infection and serum antibodies among Korean women

Table 4
Odds ratios and 95% confidence intervals according to risk factors of seropositivity for low-risk HPV (HPV 6 or 11) in Korean women

*Adjusted for age, new sexual partner in recent 6 months, lifetime number of sexual partners, age at sexual debut, tobacco use, history of STDs, oral contraceptive use, marital status, education level, and childbirth. †Single, divorced, separated or widowed. HPV, human papillomavirus; OR, odds ratio; CI, confidence interval; STDs, sexually transmitted diseases.
References
1. Moscicki AB. Impact of HPV infection in adolescent populations. J Adolesc Health. 2005. 37:S3–S9.
2. Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006. 24:Suppl 3. S3/35–S3/41.
3. Bosch FX, de Sanjose S. Chapter 1: Human papillomavirus and cervical cancer--burden and assessment of causality. J Natl Cancer Inst Monogr. 2003. 3–13.
4. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999. 189:12–19.
5. Greer CE, Wheeler CM, Ladner MB, Beutner K, Coyne MY, Liang H, Langenberg A, Yen TS, Ralston R. Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts. J Clin Microbiol. 1995. 33:2058–2063.
6. Koutsky LA, Galloway DA, Holmes KK. Epidemiology of genital human papillomavirus infection. Epidemiol Rev. 1988. 10:122–163.
7. Wiley D, Masongsong E. Human papillomavirus: the burden of infection. Obstet Gynecol Surv. 2006. 61:S3–S14.
8. Ho GY, Studentsov YY, Bierman R, Burk RD. Natural history of human papillomavirus type 16 virus-like particle antibodies in young women. Cancer Epidemiol Biomarkers Prev. 2004. 13:110–116.
9. Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: a systematic review of the literature. J Infect Dis. 2006. 194:1044–1057.
10. Shin HR, Franceschi S, Vaccarella S, Roh JW, Ju YH, Oh JK, Kong HJ, Rha SH, Jung SI, Kim JI, et al. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis. 2004. 190:468–476.
11. Sukvirach S, Smith JS, Tunsakul S, Munoz N, Kesararat V, Opasatian O, Chichareon S, Kaenploy V, Ashley R, Meijer CJ, et al. Population-based human papillomavirus prevalence in Lampang and Songkla, Thailand. J Infect Dis. 2003. 187:1246–1256.
12. Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, Galloway DA. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000. 181:1911–1919.
13. Castle PE, Wacholder S, Lorincz AT, Scott DR, Sherman ME, Glass AG, Rush BB, Schussler JE, Schiffman M. A prospective study of high-grade cervical neoplasia risk among human papillomavirus-infected women. J Natl Cancer Inst. 2002. 94:1406–1414.
14. Clifford GM, Shin HR, Oh JK, Waterboer T, Ju YH, Vaccarella S, Quint W, Pawlita M, Franceschi S. Serologic response to oncogenic human papillomavirus types in male and female university students in Busan, South Korea. Cancer Epidemiol Biomarkers Prev. 2007. 16:1874–1879.
15. Shin HR, Lee DH, Herrero R, Smith JS, Vaccarella S, Hong SH, Jung KY, Kim HH, Park UD, Cha HS, et al. Prevalence of human papillomavirus infection in women in Busan, South Korea. Int J Cancer. 2003. 103:413–421.
16. Opalka D, Lachman CE, MacMullen SA, Jansen KU, Smith JF, Chirmule N, Esser MT. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol. 2003. 10:108–115.
17. Dias D, Van Doren J, Schlottmann S, Kelly S, Puchalski D, Ruiz W, Boerckel P, Kessler J, Antonello JM, Green T, et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol. 2005. 12:959–969.
18. Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, Markowitz LE. Prevalence of HPV infection among females in the United States. JAMA. 2007. 297:813–819.
19. Herrero R, Hildesheim A, Bratti C, Sherman ME, Hutchinson M, Morales J, Balmaceda I, Greenberg MD, Alfaro M, Burk RD, Wacholder S, Plummer M, Schiffman M. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2000. 92:464–474.
20. Lee YH, Choe JY, Park SH, Park YW, Lee SS, Kang YM, Nam EJ, Park W, Kwon SR, Bae SC, et al. Prevalence of human papilloma virus infections and cervical cytological abnormalities among Korean women with systemic lupus erythematosus. J Korean Med Sci. 2010. 25:1431–1437.
21. Hankins C, Coutlee F, Lapointe N, Simard P, Tran T, Samson J, Hum L. Canadian Women's HIV Study Group. Prevalence of risk factors associated with human papillomavirus infection in women living with HIV. CMAJ. 1999. 160:185–191.
22. Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006. 24:S1–S15.
23. Goodman MT, Shvetsov YB, McDuffie K, Wilkens LR, Zhu X, Thompson PJ, Ning L, Killeen J, Kamemoto L, Hernandez BY. Prevalence, acquisition, and clearance of cervical human papillomavirus infection among women with normal cytology: Hawaii Human Papillomavirus Cohort Study. Cancer Res. 2008. 68:8813–8824.
24. Skjeldestad FE, Mehta V, Sings HL, Ovreness T, Turpin J, Su L, Boerckel P, Roberts C, Bryan J, Jansen KU, et al. Seroprevalence and genital DNA prevalence of HPV types 6, 11, 16 and 18 in a cohort of young Norwegian women: study design and cohort characteristics. Acta Obstet Gynecol Scand. 2008. 87:81–88.
25. Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003-2004. J Infect Dis. 2009. 200:1059–1067.
26. Newall AT, Brotherton JM, Quinn HE, McIntyre PB, Backhouse J, Gilbert L, Esser MT, Erick J, Bryan J, Formica N, et al. Population seroprevalence of human papillomavirus types 6, 11, 16, and 18 in men, women, and children in Australia. Clin Infect Dis. 2008. 46:1647–1655.
27. Carter JJ, Koutsky LA, Wipf GC, Christensen ND, Lee SK, Kuypers J, Kiviat N, Galloway DA. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J Infect Dis. 1996. 174:927–936.
28. Munoz N, Manalastas R Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, Clavel C, Luna J, Myers E, Hood S, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24-45 years: a randomised, double-blind trial. Lancet. 2009. 373:1949–1957.
29. Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol. 2001. 8:209–220.
30. Stanley M. Immune responses to human papillomavirus. Vaccine. 2006. 24:S16–S22.
31. Hariri S, Dunne EF, Sternberg M, Unger ER, Meadows KS, Karem KL, Markowitz LE. Seroepidemiology of human papillomavirus type 11 in the United States: results from the third National Health And Nutrition Examination Survey, 1991--1994. Sex Transm Dis. 2008. 35:298–303.
32. Kim YJ, Kim KT, Kim JH, Cha SD, Kim JW, Bae DS, Nam JH, Ahn WS, Choi HS, Ng T, et al. Vaccination with a human papillomavirus (HPV)-16/18 AS04-adjuvanted cervical cancer vaccine in Korean girls aged 10-14 years. J Korean Med Sci. 2010. 25:1197–1204.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download