Abstract
A collision tumor is defined by the presence of two separate masses in one organ, which are pathologically distinct. We described a 70-yr-old patient who complained of abnormal vaginal bleeding with a collision tumor of the uterine corpus. The patient received total hysterectomy, bilateral salphingo-oophorectomy, bilateral pelvic-paraaortic lymphadenectomy, omentectomy, and intraperitoneal chemotherapy. The uterine corpus revealed three separate masses, which were located at the fundus, anterior and posterior wall. Each tumor revealed three pathologically different components, which were malignant mixed müllerian tumor, papillary serous carcinoma, and endometrioid adenocarcinoma. Among these components, only the papillary serous carcinoma component invaded the underlying myometrium and metastasized to the regional lymph node. Adjuvant chemotherapy and radiation therapy were performed. The patient is still alive and has been healthy for the last 8 yr. We have reviewed previously reported cases of collision tumors which have occurred in the uterine corpus.
A collision tumor is defined by the coexistence of two adjacent, but histologically distinct tumor components. This tumor is considered a multiple synchronous tumor in a single organ, because these components are separated from each other by stroma without histologic admixture (1). Collision tumors have been reported in various organs, such as esophagus, stomach, colon, lung, skin, thyroid gland, breast, ovary, and uterus (2). Previously reported uterine collision tumors are mainly composed of two different histological components; serous or endometrioid adenocarcinoma, and sarcoma or neuroendocrine tumor (2-8). In this report, we describe the clinicopathologic features of an unusual uterine collision tumor. The tumor is composed of three distinct histologic components, endometrioid adenocarcinoma, papillary serous carcinoma, and malignant mixed müllerian tumor.
On November 14, 2003, a 70-yr-old female visited our hospital due to abnormal uterine bleeding and abdominal pain. Her gynecologic history included seven full-term pregnancies, six normal deliveries, one still birth, and two abortions. Menarche had occurred at the age of 17 yr and menstruation was on a regular basis. Menopause occurred at the age of 50 yr, and she has never received hormone replacement therapy. Past medical history includes hypertension, controlled by medication for the past 15 yr. She has no family history of cancer. Pelvic examination disclosed a normal vagina, unremarkable cervix, and an enlarged uterus. Transvaginal ultrasonography revealed a 9.2 × 5.9 cm-sized mass with a mixed echogenic shadow in the uterus, suggesting an endometrial malignancy. Pelvic computed tomography displayed a large mass, which occupied the entire endometrial cavity with suspicious infiltration to the myometrium and parametrial soft tissue. The adjacent organs, such as urinary bladder, rectum, and sigmoid colon, were free of tumor extension. However, the nodular infiltration of omentum was noted, suggesting the possible omental seeding. Magnetic resonance imaging revealed poorly enhancing inhomogeneous mass within the uterine cavity with tumor infiltration around the uterus with no definitive evidence of lymphadenopathy or extension to the adjacent organs. The laboratory test was within normal limit for routine blood count, electrolytes, and chemistry. The tumor marker, CA125, was elevated to 1,132 U/mL (reference range 0.1-35 U/mL). On November 17, 2003, exploratory laparotomy was performed. There was no evidence of distant metastasis on the surface of liver, spleen, diaphragm and peritoneum. However, suspicious metastatic nodules were found on omentum and the surface of sigmoid colon. The patient underwent total abdominal hysterectomy with bilateral salphingo-oophorectomy, total omentectomy, removal of the mass on the surface of sigmoid colon, bilateral pelvic and para-aortic lymph node dissection, and appendectomy. Intraperitoneal chemotherapy with paclitaxel was performed during operation due to the suspicious metastatic nodules on omentum and serosa of sigmoid colon. Adjuvant chemotherapy (paclitaxel 175 mg/m2, epirubicin 60 mg/m2, and carboplatin AUC 6, every 3 weeks, 6 cycles) and radiation therapy (55 Gy, 6 weeks) were also performed. She had been given non-steroidal aromatase inhibitor (Letrozole) orally for maintenance therapy, and remained healthy with no evidence of recurrence for 8 yr after surgery.
On gross examination, the uterus was enlarged and measures 12 × 6.5 × 6 cm in dimensions and 325 gm in weight. The outer surface was smooth and glistening without adhesion to adjacent soft tissue. On opening of the uterus, there were three separated masses arising from the endometrium (Fig. 1). The first was a large pedunculated polypoid mass at the anterior wall of the corpus, measuring 8 × 7 × 4.7 cm in dimensions. The surface of the first tumor displayed multifocal hemorrhage and necrotic friable debris, and the cut surface showed solid and fish-flesh appearance. The second was a broad based and protruding mass at the fundus of the corpus, measuring 5 × 4 × 1 cm in dimensions. By sectioning the second tumor, a cut surface of a grayish white solid mass was revealed, which invaded one half of the myometrium. The third was an irregular elevated mass-like lesion, measuring 2.5 × 1.3 × 1 cm in dimensions. The cut surface of this lesion showed a grayish white solid mass without definitive myometrial invasion. The histologic findings and immunohistochemical staining results of these tumors revealed a malignant mixed müllerian tumor, papillary serous carcinoma, and endometrial adenocarcinoma, respectively (Fig. 2). Among these components, only the serous carcinoma component revealed invasive growth, while the other two components were confined to the endometrium. The findings of pathologic examination and immunohistochemistry are summarized in Table 1. Pelvic and para-aortic lymph nodes showed metastasis of the papillary serous carcinoma component. Biopsied tissue from the serosal lesion of the sigmoid colon showed seeding of papillary serous carcinoma. Both ovaries and salpinx were unremarkable. Collectively, the FIGO stage was IIIc.
Tumors with a combination of different histology are divided into two clinicopathologic groups, collision or composite tumors (9). The collision tumor has more than two juxtapositioned masses and each mass displays a different histology. In a collision tumor, each mass has a distinct boundary and is separated by non-neoplastic stroma. In contrast, the intermingling of more than two different components in one tumor mass is designated as a composite tumor. The well-known example is a malignant mixed müllerian tumor of the uterus. The present case has three separate masses located at the fundus, anterior and posterior wall of the uterine corpus, with different histology and immunophenotype. Collision tumors of two distinct components have already been reported. However, to the best of our knowledge, a collision tumor with three distinct components has never been reported.
The clinicopathologic characteristics of the previously reported and present uterine collision tumors are summarized in Table 2. The mean age of diagnosis was 66.1 yr (range, 47-85 yr). A total of 10 patients had 21 separate tumors in the uterine corpus. The most frequent histologic type was endometrial adenocarcinoma (10/21, 47.6%), followed by homologous and heterologous sarcomas (4/21, 19.0%), endometrial stromal sarcoma (3/21, 14.2%), malignant mixed müllerian tumor (2/21, 9.5%), small cell carcinoma (1/21, 4.7%), and hepatoid carcinoma (1/21, 4.7%). The patients had received total hysterectomy and bilaterial salpingooophorectomy with or without pelvic-paraaortic lymph node dissection. Adjuvant chemotherapy and/or radiation therapy was performed on three patients (case 6, 7, 10). One patient (case 4) underwent neoadjuvant chemotherapy before surgical resection. There is no information on adjuvant treatment on any other patients. FIGO stage ranged from Ib to IIIc, and most patients had a tumor with an advanced stage. The follow-up information was obtained from 7 patients. Four patients died in 4 to 18 months (mean, 8.2 months) after initial diagnosis. The deceased patients had tumors with high FIGO stage, two patients with FIGO stage IIIb and the others with FIGO stage IIIc. On the other hand, two patients who had a relatively lower FIGO stage remained alive up to the last follow-up period. The patient (case 7) with FIGO stage Ib survived for 6.5 yr and the patient (case 6) with FIGO stage IIIa, survived for 1.5 yr. Regardless of histologic subtype, the clinical outcome generally depended on tumor stage. In contrast to previous cases, the present case presents a patient with a collision tumor of FIGO stage IIIc, who remained disease-free for 8 yr after the initial diagnosis. The favorable prognosis of this case may be due to the multimodal treatment performed on the patient. Although advanced metastatic diseases generally have a poor survival rate, aggressive surgeries and adjuvant therapies may increase such survival rates.
On reviewing the previously reported cases, pathologic information on myoinvasive components was found in 8 cases. Among these cases, 4 (case 2, 5, 6, 7) demonstrated invasion of only the sarcoma component into the myometrium, whereas the carcinoma component was confined to the endometrium. These cases had endometrioid adenocarcinomas as the carcinoma component, known to have a relatively favorable prognosis (10). Invasive and metastatic components of collision tumors are supposed to be dependent on their original biologic behavior. This explanation is supported by cases presenting high-grade carcinomas collided with malignant mixed müllerian tumors. High-grade carcinomas, such as serous (case 10) and hepatoid carcinomas (case 8), display myomterial invasion, whereas malignant mixed müllerian tumors are confined to the endometrium. These pathologic findings suggest that each component of collision tumors occur coincidentally with no connection, and the biologic behavior depends on their own tumor characteristics.
The preoperative diagnosis of collision tumor is difficult when these tumor components are closely located. In our case, the radiologic studies could not discriminate three masses. The possible explanation includes that one mass was dominantly larger than the other two, and all these tumors were arising in the endometrium and faced each other in uterine cavity. Careful pathologic examination provides exact tumor stage for each tumor and histologic subtype, which can lead to predict correct clinical behavior.
Figures and Tables
Fig. 1
Macroscopic appearance and schematic view of the hysterectomy specimen. (A) The opened uterus reveals a large polypoid mass in the anterior wall and a broad-based protruding mass in fundus. (B) The posterior wall of uterine corpus shows a slightly elevated mass. (C, D) Schematic view of three separate tumors with their pathologic diagnosis.
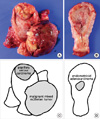
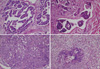
Fig. 2
Histopathology of the hysterectomy specimen HE stained. (A) Papillary serous carcinoma, which is found at fundus of uterine corpus, consists of pleomorphic tumor cells with papillary growth pattern. (B) The lymphovascular invasion is present at the periphery of the papillary serous carcinoma. (C) Section from posterior wall consists of endometrioid adenocarcinoma, showing glandular and solid growth pattern. (D) Section from polypoid mass reveals malignant mixed müllerian tumor, consisting of carcinomatous and sarcomatous components.
References
1. Van Eeden S, Nederlof PM, Taal BG, Offerhaus GJ, Van Velthuysen ML. A tumour with a neuroendocrine and papillary serous component: two or a pair? J Clin Pathol. 2002. 55:710–714.
2. Lam KY, Khoo US, Cheung A. Collision of endometrioid carcinoma and stromal sarcoma of the uterus: a report of two cases. Int J Gynecol Pathol. 1999. 18:77–81.
3. Gaertner EM, Farley JH, Taylor RR, Silver SA. Collision of uterine rhabdoid tumor and endometrioid adenocarcinoma: a case report and review of the literature. Int J Gynecol Pathol. 1999. 18:396–401.
4. Lifschitz-Mercer B, Czernobilsky B, Dgani R, Dallenbach-Hellweg G, Moll R, Franke WW. Immunocytochemical study of an endometrial diffuse clear cell stromal sarcoma and other endometrial stromal sarcomas. Cancer. 1987. 59:1494–1499.
5. Patwardhan JR, Gadgil RK. Collision tumour of the uterus. Indian J Cancer. 1969. 6:194–197.
6. Shaco-Levy R, Manor E, Piura B, Ariel I. An unusual composite endometrial tumor combining papillary serous carcinoma and small cell carcinoma. Am J Surg Pathol. 2004. 28:1103–1106.
7. Sreenan JJ, Hart WR. Carcinosarcomas of the female genital tract. A pathologic study of 29 metastatic tumors: further evidence for the dominant role of the epithelial component and the conversion theory of histogenesis. Am J Surg Pathol. 1995. 19:666–674.
8. Takahashi Y, Inoue T. Hepatoid carcinoma of the uterus that collided with carcinosarcoma. Pathol Int. 2003. 53:323–326.
9. Lewin K. Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol. 1987. 11:71–86.
10. Rose PG. Endometrial carcinoma. N Engl J Med. 1996. 335:640–649.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download