Abstract
The mechanism of chronic pain is very complicated. Memory, pain, and opioid dependence appear to share common mechanism, including synaptic plasticity, and anatomical structures. A 48-yr-old woman with severe pain caused by bone metastasis of breast cancer received epidural block. After local anesthetics were injected, she had a seizure and then went into cardiac arrest. Following cardiopulmonary resuscitation, her cardiac rhythm returned to normal, but her memory had disappeared. Also, her excruciating pain and opioid dependence had disappeared. This complication, although uncommon, gives us a lot to think about a role of memory for chronic pain and opioid dependence.
The mechanism of chronic pain is not yet completely understood. The main structures that are known to process pain are the primary and secondary somatosensory, insula, anterior cingulated, and prefrontal cortices, as well as the thalamus (1). Additional structures that play a smaller role are the basal ganglia, cerebellum, amygdala, hippocampus, and areas within the parietal and temporal cortices (1). These complex brain structures integrate nociceptive input with contextual information and memory to provide cognitive mediation of pain affect (1). Additionally, opioid dependence is related to pathologic learning and memory (2). We examined the case of a patient who experienced excruciating pain caused by bone metastasis of breast cancer and who became pain-free, experienced amnesia, and recovered from opioid dependence after cardiopulmonary resuscitation (CPR).
There are few reports concerning the interrelationship between pain, memory, and drug dependence; hence, we are reporting a case of amnesia and pain relief with a literature review.
A 48-yr-old woman who was diagnosed with breast cancer 5 yr ago was transferred to our pain clinic for pain management on April 21, 2010. She had undergone a modified radical mastectomy, chemotherapy, and radiotherapy. Cancer had spread to the cerebral meninges, bones, and liver. The patient experienced excruciating pain in her back and in her right lower chest area (Fig. 1). The pain had started 6 months earlier and became aggravated 2 months before admission. Her visual analog scale (VAS) score was 9-10/10, and she had received morphine (300 mg/day, intravenous administration). She had strong dependence on morphine. She always wanted morphine before every movement.
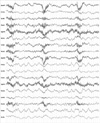
We performed a single epidural block as a trial for predicting its effect before a planned continuous epidural block using an epidural port. The epidural block was performed in the left lateral position. A 22-gauge Tuohy needle was inserted blindly into the lumbar epidural space at the L1-2 level. We used the loss-of-resistance (LOR) technique to detect the proper epidural space. After observing no leakage of cerebrospinal fluid (CSF) and blood, we slowly injected 0.4% mepivacaine hydrochloride (8 mL), without a test dose of the drug. Approximately 1 min later, the patient had a sudden seizure of generalized tonic-clonic type for 30 s, and then lost consciousness and demonstrated difficulty breathing. Immediately, artificial respiration was performed on her. At that time, her blood pressure (BP) level was 100/80 mmHg, and her heart rate (HR) was 60 beats/min. We immediately attached monitoring devices to her body. The patient was intubated with an endotracheal tube, mechanically ventilated, and her condition was monitored closely. Later, she went into cardiac arrest. We performed CPR for 5 min until her cardiac rhythm returned to normal. Her vital signs were as follows: BP, 110/50 mmHg; HR, 120 beats/min; body temperature, 36.5℃; respiratory rate, 20/min; SpO2, maintained at 100%. A neurologic assessment by a neurologist did not reveal any neurological abnormalities other than unconsciousness. Brain computed tomography (CT) scan did not show any specific abnormal findings. We performed brain magnetic resonance imaging (MRI) (Fig. 2) twice, but neither scan showed any abnormalities except metastatic lesions in the meninges and bones. Electroencephalography (EEG) (Fig. 3) showed a partial seizure lesion and severe, diffuse cerebral dysfunction in the right temporal lobe area. After 14 days, we performed another EEG again, which showed same results. Anticonvulsants were not administered because she did not have seizure any more. She was transferred to the intensive care unit. Seven days later, she opened her eyes in response to her name. She made eye contact with the medical team, grasped her hand, and responded to the doctor's requests. After 16 days, we removed her endotracheal tube. After 17 days, she could have simple conversations with her husband, and started communicating fluently after 22 days. Although no neurologic sequelae remained, she could not remember her 3 yr of autobiographic memory prior to the incident, and the severe pain of which she had complained had almost disappeared (VAS 1/10). Opioid withdrawal symptoms were not observed. Morphine was no longer needed. She was moved to a general ward.
After 30 days, her right humerus was fractured because of metastasis. She again experienced extreme pain (VAS 7-8/10), but did not feel any pain in her back or right lower chest where she had experienced pain before. She passed away 2 months later.
Pain is a very complicated neurophysiological phenomenon (1-3). Although many studies have been performed to demonstrate this complicated phenomenon, it is still poorly understood.
In the present case, the patient did not show any neurological complications except memory loss. Memory loss can be classified according to many criteria; this patient demonstrated retrograde amnesia, particularly remote and episodic amnesia. This generalized and long-lasting episodic memory loss is almost always related to bilateral injury of the limbic network in the thalamus or the hippocampo-entorhinal complex (4). Most cases of amnesia are not caused by injury of the memory storage area, but usually by dysfunction of the limbic network, which composes pieces of stored memories from many different parts of brain into coherent events. Many factors can damage the hippocampus. The most common causes are Alzheimer's disease; traumatic injury; vascular infarction; infection; and metabolic insults including hypoxia, hypoglycemia, and prolonged seizures (5). Among these various causes, we assume that the causes of amnesia in the present case are hypoxic brain injury and prolonged seizure after respiratory and cardiac arrest. Many cognitive impairments and memory losses occur by hypoxic brain injury after cardiac arrest, but the precise mechanism of this phenomenon is not yet clearly proven (6). Fleiss et al. (7) reported that hypoxic brain injury in an animal model of birth asphyxia produced significant functional deficits in the hippocampus, and caused a reduction of long-term potentiation (LTP), paired pulse facilitation (PRF), and post-tetanic potentiation (PTP). This occurred in the absence of gross cellular damage. In a positron emission tomographic study of 2 post-ischemic-hypoxic amnesia patients, there was destruction of the inhibitory pathways to the thalamus and basal ganglia (8). Amnesia is not related to the duration of cardiac arrest (9). Hence, we believe that a short arrest duration, as in our case, may be sufficient to cause memory loss.
Epilepsy, especially initiated from the temporal lobe, which is related to memory, can cause memory loss. Transient epileptic amnesia, accelerating long-term forgetting, and remote memory loss commonly occur after epilepsy (10). Although our patient did not have any history of epilepsy, a temporary seizure occurred after the epidural block. In addition, EEG showed a partial seizure of the temporal lobe area; hence, we presume that seizure is another possible cause of amnesia. In an animal model of hypoxia-induced seizures (HS), seizure had an effect on the synaptic plasticity, especially diminish silent synapses and LTP in hippocampus (11).
In this case, both memory and severe pain disappeared without any neurologic sequelae. We do not know the exact reason of this phenomenon, but are assuming that the pain may be gone due to loss of memory which plays important role in chronic pain. Recent studies reveal an interrelationship between chronic pain and the functional, anatomical, and chemical reorganization of the brain. Memories of nociceptive stimuli can lead to persistent pain coding in the brain, and these are associated with central mechanisms, and cortical reorganization in the brain (12-14). Both the intensity and chronicity of pain accentuate the development of pain memory (14). Limbic areas are important structures for pain memory. The amygdala is activated during pain, and it plays a role as a "defensive behavioral mechanism" that controls the transmission of the nociceptive experience to the brain; this has relevance to the memory storage of past pain experiences and their context. Moreover, the amygdala is important for fear conditioning (15). The hippocampus does not appear to have a direct role in the formation of pain or fear avoidance, but is associated with learning pain-related behavior (15, 16). Recent neuroimaging studies have demonstrated that the anterior cingulate cortex (ACC) is closely related to the affective features of pain, such as unpleasantness, and has a multimodal integrative, rather than a specific, nociceptive role (15, 17). There is also an association between pain and the posterior cingulate cortex (PCC) (18). Nielsen et al. (13) have performed a meta-analysis of the segregation between memory and pain components. They find that the distribution of memory and pain brain activations in the PCC is quite similar.
Opioid dependence is generally regarded as a disease associated with learning and memory. Abuse of drugs for a long time cause changes in the brain, and induce memory formation (19). Neural plasticity is induced by chronic substance consumption, and this is related to drug-induced LTP and synaptic changes in the mesolimbic system. This changes are also implicated in memory formation (2, 19, 20). Opioid dependence, learning, and memory also share the same complex circuits involving the hippocampus, cerebral cortex, ventral and dorsal striatum, and amygdala (19). In the present case, the patient was prescribed with high doses of morphine. Nevertheless, the patient demanded supplemental morphine whenever she tried to move. However, after she experienced a cardiac arrest, she no longer required morphine.
Choi et al. (3) report 2 patients who experienced pain reduction and improvement of opioid dependence after sudden memory loss. Their reports are quite similar to ours. Their 2 patients experienced sudden memory loss due to status epilepticus and minor brain injury, respectively. One of their reported patients could recall his episodic autobiographical memory, and he began to feel pain again.
In conclusion, we report a case of pain relief and recovery from opioid dependence associated with memory loss. In this case, we do not clearly know the cause of memory loss, pain relief, and the disappearance of opioid dependence. We also do not know why the patient's most painful memory, of all possible memories, was affected. However, we assume that memory loss plays a major role in the modulation of both pain and opioid use. Moreover, understanding this complex mechanism in the brain can help treat intractable pain and addiction, which will allow us to help patients forget their chronic pain in the future.
Figures and Tables
Fig. 1
Radiography of the patient. A radiographic view shows multiple pathologic fractures on the vertebra and right 10th and 11th ribs.

References
1. Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000. 288:1769–1772.
2. Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005. 162:1414–1422.
3. Choi DS, Choi DY, Whittington RA, Nedeljković SS. Sudden amnesia resulting in pain relief: the relationship between memory and pain. Pain. 2007. 132:206–210.
4. Mesulam M. Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Aphasia, momory loss, and other focal cerebral disorders. Harrison's principles of internal medicine. 2011. 18th ed. New York: McGraw-Hill;202–212.
5. Squire LR, Zola SM. Ischemic brain damage and memory impairment: a commentary. Hippocampus. 1996. 6:546–552.
6. Bass E. Cardiopulmonary arrest. Pathophysiology and neurologic complications. Ann Intern Med. 1985. 103:920–927.
7. Fleiss B, Coleman HA, Castillo-Melendez M, Ireland Z, Walker DW, Parkington HC. Effects of birth asphyxia on neonatal hippocampal structure and function in the spiny mouse. Int J Dev Neurosci. 2011. 29:757–766.
8. De Reuck J, Vanwalleghem I, Hemelsoet D, De Weweire M, Strijckmans K, Lemahieu I. Positron emission tomographic study of post-ischaemic-hypoxic amnesia. Eur Neurol. 2003. 49:131–136.
9. O'Reilly SM, Grubb NR, O'Carroll RE. In-hospital cardiac arrest leads to chronic memory impairment. Resuscitation. 2003. 58:73–79.
10. Butler CR, Zeman AZ. Recent insights into the impairment of memory in epilepsy: transient epileptic amnesia, accelerated long-term forgetting and remote memory impairment. Brain. 2008. 131:2243–2263.
11. Zhou C, Lippman JJ, Sun H, Jensen FE. Hypoxia-induced neonatal seizures diminish silent synapses and long-term potentiation in hippocampal CA1 neurons. J Neurosci. 2011. 31:18211–18222.
12. Niv D, Devor M. Chronic pain as a disease in its own right. Pain Pract. 2004. 4:179–181.
13. Katz J, Melzack R. Pain 'memories' in phantom limbs: review and clinical observations. Pain. 1990. 43:319–336.
14. Flor H. Painful memories. Can we train chronic pain patients to 'forget' their pain? EMBO Rep. 2002. 3:288–291.
15. Lorenz J, Hauck M. Fishman SM, Ballantyne JC, Rathmell JP, editors. Supraspinal mechanisms of pain and nociception. Bonica's management of pain. 2010. 4th ed. Baltimore: Lippincott Williams & Wilkins;61–73.
16. Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986. 6:2950–2967.
17. Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005. 6:533–544.
18. Nielsen FA, Balslev D, Hansen LK. Mining the posterior cingulate: segregation between memory and pain components. Neuroimage. 2005. 27:520–532.
19. Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem. 2002. 78:637–647.
20. von der Goltz C, Kiefer F. Learning and memory in the aetiopathogenesis of addiction: future implications for therapy? Eur Arch Psychiatry Clin Neurosci. 2009. 259:S183–S187.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download