Abstract
This study was performed to assess the usefulness of magnetoencephalography (MEG) as a presurgical evaluation modality in Korean pediatric patients with lesional localization-related epilepsy. The medical records and MEG findings of 13 pediatric patients (6 boys and 7 girls) with localization-related epilepsy, who underwent epilepsy surgery at Seoul National University Children's Hospital, were retrospectively reviewed. The hemispheric concordance rate was 100% (13/13 patients). The lobar or regional concordance rate was 77% (10/13 patients). In most cases, the MEG spike sources were clustered in the proximity of the lesion, either at one side of the margin (nine patients) or around the lesion (one patient); clustered spike sources were distant from the lesion in one patient. Among the patients with clustered spike sources near the lesion, further extensions (three patients) and distal scatters (three patients) were also observed. MEG spike sources were well lateralized and localized even in two patients without focal epileptiform discharges in the interictal scalp electroencephalography. Ten patients (77%) achieved Engel class I postsurgical seizure outcome. It is suggested that MEG is a safe and useful presurgical evaluation modality in pediatric patients with lesion localization-related epilepsy.
Magnetoencephalography (MEG) records the extracranial magnetic field generated by the electrical activity of the brain (1). Because MEG records continuous magnetic activity through multiple sensors that cover the entire brain, it has excellent temporal and spatial resolution compared with other modalities used to evaluate epilepsy. Important advantages of MEG over electroencephalography (EEG) are that the magnetic field detected from the scalp is not distorted or attenuated (2) and that accurate localization of the epileptic spike source is possible. Overlaying this localized spike source in the patient's structural imaging (magnetic source imaging, MSI) provides valuable information about the localization of the epileptogenic foci (3). The usefulness of MEG as a presurgical evaluation tool has been demonstrated in previous studies. The accuracy of MEG spike source localization was satisfactory in a study of 455 patients (4). MEG spike sources localized well in temporal and extratemporal lesional epilepsy, and sometimes provided additive localization information to brain MRI or interictal scalp EEG (5). MEG also added diagnostic yield to scalp video-EEG (6, 7).
Although the usefulness of MEG has been well demonstrated and its application has yielded numerous promising results, the superiority of MEG over EEG is not widely accepted. Moreover, there is no solid evidence that MEG can be substituted for invasive intracranial EEG in epilepsy surgery (8). Although the number of reports on the use of MEG in pediatric epilepsy surgery in Asia is increasing, there is no report on the use of MEG in Korean pediatric patients with epilepsy. We examined the usefulness of MEG in evaluating Korean pediatric patients with lesional localization-related epilepsy. We also sought to analyze the pattern of MEG spike source distribution in relation to the lesions found by brain magnetic resonance imaging (MRI) and the surgical outcomes.
Of 41 pediatric patients who underwent MEG spontaneous magnetic activity analysis at the MEG Center of Seoul National University Hospital between May 2005 and December 2010, 13 patients (6 boys and 7 girls) received epilepsy surgery for the diagnosis of lesional localization-related epilepsy and were included in the analysis. The electronic medical records were reviewed retrospectively. Data about sex, age, onset age, age at MEG examination, seizure semiology, pre- and post-operative seizure frequency, brain MRI, brain fluorodeoxyglucose positron emission tomography (FDG PET) and the pathology report were reviewed.
A 128-channel Grass Telefactor® digital EEG was used for the interictal scalp and long-term video-EEG monitoring. Scalp electrodes were placed according to the international 10-20 system. If necessary, electromyography (EMG) electrodes were also placed during the long-term video-EEG monitoring. Interictal EEGs, video-EEGs, and intracranial EEGs were reviewed independently by three pediatric epileptologists. The seizure semiology was described based either on the parents' or the caregiver's reports or on the video-EEG monitoring findings. The seizure semiology description and classification were based on the International League Against Epilepsy classification of epileptic seizures (9, 10). The sampling rate was 200 Hz for all recordings.
Spontaneous brain magnetic activity was recorded using a helmet-shaped, 306-channel, whole-head neuromagnetometer (VectorView™; Elekta Neuromag Oy, Helsinki, Finland). The MEG sensors comprised 2 planar gradiometers and 1 magnetometer. The recording time was 50-90 min and most of the recordings comprised of spontaneous or sedated sleep records. The sampling rate for data acquisition was 600 Hz. A band-pass filter (0.1-200 Hz) and a notch filter (60 Hz) were applied. Chloral hydrate (50 mg/kg; maximum, 1,500 mg) was used when conscious sedation was required for infants and preschool-aged patients.
The MEG recordings were analyzed and the spike sources were localized using the equivalent current dipole method and current density imaging. The data was processed using US FDA-approved Neuromag software (Elekta Neuromag Oy, Helsinki, Finland). MEG spike sources with goodness of fit values > 85% were considered significant. When more than half of the sources were localized to the hemisphere with MRI lesions, the results were considered concordant. Similarly, when more than half of the sources were localized to a certain lobe or the anatomical region of MRI abnormality, the results were considered concordant. MEG spike sources were classified as clustered around the lesion with or without extension, clustered distant from the lesion, or scattered.
The postsurgical seizure frequency noted in the outpatient visit was reviewed and was classified according to Engel's classification (11).
This study protocol was reviewed and approved by the institutional review board (IRB) of the Seoul National University Hospital (IRB No.H-1111-052-386). Waiver of informed consent was approved by the IRB after reviewing the study design, which was a retrospective review of medical record and MEG data.
The mean age at the onset of epilepsy was 6.4 yr (range, 10 months-12.1 yr) and mean age at the examination was 8.4 yr (range, 2.8-13.0 yr). The preoperative seizure frequency ranged from multiple daily seizures to monthly seizures. The most common seizure semiology was dialeptic or hypomotor seizure, followed by versive, focal tonic, focal clonic, hypermotor, and generalized tonic-clonic seizures. The most common location of MRI lesion was the temporal lobe (n = 8; six medial and two lateral), followed by frontal lobe (n = 3) and occipital lobe (n = 2) (Table 1).
The hemispheric concordance rate was 100% (13/13 patients) and the lobar or regional concordance rate was 77% (10/13 patients). The number of MEG spikes analyzed was 2-52 (mean, 18.8). More than eight spikes were analyzed in 10 patients, but only a few spikes were recorded in two patients (patients 3 and 13). With regard to distribution and location, the MEG spike sources were clustered around or in proximity to the MRI lesion in 10 patients (77%). Among these patients, the MEG spike sources were extended from the cluster in three patients and distant scatters coexisted in another three patients. The MEG spike sources were scattered in two patients and were clustered distant from the lesion in one patient (Table 2).
MEG spike source clusters were observed in 11 patients, and these clusters were located in the proximity of the lesion in 10 patients. In most patients, the MEG spike sources were located on the margin of the lesion: superior (patients 4, 5, and 6), lateral (patients 1, 7, and 8), and superior and lateral (patients 10, 11, and 12). In patient 2, the spike sources were located around the lesion (Fig. 1). Among these patients, distant scatters were also identified in the contralateral hemisphere in two patients and ipsilateral hemisphere in one patient (Fig. 2). The MEG spike source cluster was distant from the lesion in patient 3. In the two patients without clusters, the MEG spike sources were scattered in both hemispheres.
EEG abnormalities of epileptiform discharges or background abnormalities were present in 11 patients. Focal epileptiform discharges were present in 10 patients (77%), and the MEG spike source locations were concordant with these EEG discharges. In two patients with a normal EEGs (patients 4 and 8) and in one patient with no focal epileptiform activity (patient 1), MEG detected well-lateralized and localized MEG spike sources. MEG spike sources were concordant in eight of the 10 patients whose PET findings were abnormal. MEG findings were concordant with the ictal onset zone identified by long-term video-EEG monitoring (patients 7, 11, and 13) and invasive intracranial EEG monitoring (patients 4 and 13).
Lesionectomy was performed in all patients. Anterior temporal lobectomy was performed in two patients (patients 5 and 10). The surgeons determined the resection margin using the preoperative brain MRI findings and intraoperative ultrasonograms. An invasive intracranial EEG-based resection margin was applied in 2 patients (patients 4 and 13). The pathology of the lesions included focal cortical dysplasia (n = 7), dysembryoplastic neuroepithelial tumor (n = 4), and ganglioglioma (n = 2). The mean follow-up duration after the surgery was 39.2 months (range, 26-64 months). The postoperative seizure outcomes included Engel class I (n = 10), class II (n = 1), and class III (n = 2). Nine patients (69%) were completely seizure free (Table 2).
Identification and accurate localization of the epileptogenic area are critical for successful epilepsy surgery. In this study, the hemispheric concordance rate of the MEG spike sources was 100%. Localization was also satisfactory at the level of the anatomical region because the MEG spike sources were distributed exactly at or in the vicinity of the lesion in most patients. The hemispheric lateralization rate is similar to or higher than that reported for patients with intractable focal epilepsy in previous studies: 68% (when compared to brain MRI and intracranial ictal onset zone) (12), 82% (lobar concordance to anatomical lesional in brain MRI) (7), and 89% (when compared to the final surgical decision) (4). The lobar or regional localization rate is similar to the 72% rate reported in patients with intractable focal epilepsy (7). Although the number of patients in this study is small, our data suggest that MEG is useful in identifying the lateralization and localization of the structural lesions in pediatric epilepsy patients. We observed good outcomes of 69% of patients free from seizures and 77% of patients with an Engel class I outcome after a sufficient follow-up period. Favorable postsurgical seizure outcomes of MEG have also been shown in previous studies (7, 13, 14). Although we did not compare the MEG spike source foci with the intracranial EEG ictal onset in this study, complete seizure freedom after surgery can be considered as indirect evidence that the epileptogenic area was removed or the epileptogenic condition was resolved. These findings allow us to infer that MEG is effective in identifying the lateralization and localization of epileptogenic areas in patients with lesional epilepsy.
In a study comparing the diagnostic sensitivity of presurgical evaluation modalities, MSI had the highest sensitivity (58%-64%), as well as the highest predictive value for identifying the ictal onset zone (15). Sutherling et al. reported additional benefits of MSI for preoperative planning (16). In our study, three patients had an interictal FDG PET result showing no decrease in glucose uptake. In two of these patients (patients 1 and 2), MEG identified 13 and 20 MEG spike sources that recognized the lesional hemisphere and localized the spike source clusters. In two patients with normal interictal scalp EEGs (patients 4 and 8), MEG also identified the lesional hemisphere and the anatomical lobe that was concordant with the lesion. These patients were completely seizure free for 26-45 months after surgery. It is well known that MEG and EEG are not mutually exclusive in discriminating abnormal electrical activity (17). A recent study showed that a multimodality approach can improve surgical outcome even in pediatric patients with intractable nonlesional epilepsy (18). Although we did not collect quantitative data, we demonstrated that MEG provides additive information in patients with normal FDG PET or interictal scalp EEG results. Our findings suggest that MEG should be performed in patients undergoing presurgical evaluation, especially in those with discordant or insufficient information on lateralization or localization.
The distribution of MEG spike sources in relation to the MRI lesions varied and included clusters, clusters with extension, or scatters. The clusters were located around the lesion or at the margin of the lesion. In most patients, the clusters were located on one side of the lesion and sometimes extended further to the same side. Similar findings were reported in previous studies that showed surrounding MEG spike sources in patients with focal cortical dysplasia (13, 19, 20). Although we found no differences in the MEG spike source distribution according to different pathologies, we observed the typical distribution of clusters located at the margin of the lesion either around the lesion or at one side of the margin. This finding suggests that the irritative zone in lesional focal epilepsy may reside in the margin of the lesion, and hence indicates one possible pathophysiological mechanism underlying the epileptogenesis of lesional epilepsy. These findings should be clarified in future research with more patients and objective methodology.
This is the first study performed to assess the value of MEG in pediatric patients from the tertiary epilepsy surgery center soon after utilizing magnetoencephalography. Although we could not demonstrate the additional benefit of MEG in lesional epilepsy surgery, this was shown in previous studies. Removal of the dysplastic cortex that has not been characterized by brain imaging but by MEG was required for complete seizure freedom (13). In cases of discordant presurgical evaluation, concordant lateralization was related to good seizure control (12). Multiple MEG spike source clusters required identification of the multiple or extensive epileptogenic zone prior to accurate localization and delineation of the resection margin (21). Concordant lateralization and precise localization of interictal MEG spike sources that were noted in this study may provide additional information in the presurgical evaluation and planning in lesional epilepsy surgery. MEG spike sources demonstrate and represent interictal irritative zone, this may partly explain the poor outcome of three patients in this study. Ictal onset zone should be verified by the intracranial EEG and interictal spike sources of MEG may provide compensatory information. MEG and EEG are not mutually exclusive (17), so both modalities should be used together to achieve the best information regarding the epileptogenic zone.
Young patients were included in this study. The youngest patient reported to have undergone MEG analysis was 5 months (12), and another study included a patient who had received surgery at 4 yr of age (22). The age of patients included in the pediatric MEG studies usually range from 4 to 18 yr, and the mean age of patients were usually 10 to 12 yr (13, 20, 22). The youngest patient in this study was 2.8 yr old when she underwent MEG analysis, and other patients were 3.9, 4.1, and 4.4 yr old at the time of MEG analysis. Conscious sedation with oral chloral hydrate, instead of general anesthesia or parenteral sedatives, was sufficient for these younger children and we found no limitations in processing and analyzing the MEG data. We emphasize that MEG can be performed safely and effectively even in younger pediatric patients.
There are limitations to this study. We could not demonstrate directly that the MEG spike sources represent the ictal onset zone, and we found no difference between the MEG spike source distribution and pathology of the epileptogenic lesion. This should be verified in future studies.
In conclusion, this is the first Korean report of MEG in the field of pediatric epilepsy surgery. MEG is safe and effective for analyzing pediatric patients with lesional localization-related epilepsy. The hemispheric and regional concordance is satisfactory. The favorable surgical outcomes in this study provide indirect evidence that the irritative zone defined by MEG can provide valuable information about the epileptogenic area.
Figures and Tables
Fig. 1
Distribution and location of clustered MEG spike sources. (A) T1-weighted axial brain magnetic resonance imaging (MRI) shows MEG spike sources clustered around the margin of the lesion. Because the spike sources are overlaid in a single axial image, spike sources that are inside the lesion are located superior or inferior to the lesion (patient 2). (B) T2-weighted axial brain MRI shows MEG spike sources clustered on the margin of the lesion (overlaid image of patient 7). (C) T1-weighted axial brain MRI shows MEG spike sources clustered at the margin lateral to the lesion (overlaid image of patient 12). Epileptogenic lesion (arrow) is visible in brain MRI. R, right; L, left; A, anterior; P, posterior.
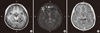
Fig. 2
MEG spike sources clustered around the lesion and scattered in patient 12. (A-C) Axial, sagittal, and coronal contrast-enhanced T1-weighted brain magnetic resonance imaging (MRI) show clusters and distant scatters (overlaid on a single brain MRI image). (D) MEG spike sources presented in an imaginary three-dimensional plane show the distribution of MEG spike sources of clusters and scatters. R, right; L, left; H, head; F, foot; AL, anterior left; PR, posterior right; A, anterior; P, posterior.

Table 2
Summary of lesion locations, interictal EEG findings, FDG PET, MEG spike source distribution, pathology, and postsurgical Engel classification of 13 patients

Number in parenthesis shows the number of magnetic spike sources located to the specific brain region. *MEG spike source cluster was located distant from the lesion. L, left; R, right; F, frontal; T, temporal; O, occipital; P, parietal; N, no abnormality; C, cluster; E, extension; S, scatter; DNT, dysembryoplastic neuroepithelial tumor; FCD, focal cortical dysplasia; GGL, ganglioglioma.
References
1. Cohen D. Magnetoencephalography: evidence of magnetic fields produced by alpha-rhythm currents. Science. 1968. 161:784–786.
2. Ricci GB, Romani GL, Salustri C, Pizzella V, Torrioli G, Buonomo S, Peresson M, Modena I. Study of focal epilepsy by multichannel neuromagnetic measurements. Electroencephalogr Clin Neurophysiol. 1987. 66:358–368.
3. Gallen CC, Hirschkoff EC, Buchanan DS. Magnetoencephalography and magnetic source imaging. Capabilities and limitations. Neuroimaging Clin N Am. 1995. 5:227–249.
4. Stefan H, Hummel C, Scheler G, Genow A, Druschky K, Tilz C, Kaltenhäuser M, Hopfengärtner R, Buchfelder M, Romstöck J. Magnetic brain source imaging of focal epileptic activity: a synopsis of 455 cases. Brain. 2003. 126:2396–2405.
5. Knowlton RC, Laxer KD, Aminoff MJ, Roberts TP, Wong ST, Rowley HA. Magnetoencephalography in partial epilepsy: clinical yield and localization accuracy. Ann Neurol. 1997. 42:622–631.
6. Pataraia E, Simos PG, Castillo EM, Billingsley RL, Sarkari S, Wheless JW, Maggio V, Maggio W, Baumgartner JE, Swank PR, et al. Does magnetoencephalography add to scalp video-EEG as a diagnostic tool in epilepsy surgery? Neurology. 2004. 62:943–948.
7. Paulini A, Fischer M, Rampp S, Scheler G, Hopfengärtner R, Kaltenhäuser M, Dörfler A, Buchfelder M, Stefan H. Lobar localization information in epilepsy patients: MEG - a useful tool in routine presurgical diagnosis. Epilepsy Res. 2007. 76:124–130.
8. Bast T. Wyllie E, Cascino G, Gidal B, editors. Magnetoencephalography. Wyllie's treatment of epilepsy: principles and practice. 2011. Philadelphia: Lippincott Williams & Wilkins;869–875.
9. Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981. 22:489–501.
10. Blume WT, Lüders HO, Mizrahi E, Tassinari C, van Emde Boas W, Engel J Jr. Glossary of descriptive terminology for ictal semiology: report of the ILAE task force on classification and terminology. Epilepsia. 2001. 42:1212–1218.
11. Engel J Jr, VanNess P, Rasmussen T, Ojemann L. Engel J, editor. Outcome with respect to epileptic seizures. Surgical treatment of the epilepsies. 1993. 2nd ed. New York: Raven Press;609–621.
12. Ochi A, Otsubo H, Iida K, Oishi M, Elliott I, Weiss SK, Kutomi T, Nakayama T, Sharma R, Chuang SH, et al. Identifying the primary epileptogenic hemisphere from electroencephalographic (EEG) and magnetoencephalographic dipole lateralizations in children with intractable epilepsy. J Child Neurol. 2005. 20:885–892.
13. Otsubo H, Ochi A, Elliott I, Chuang SH, Rutka JT, Jay V, Aung M, Sobel DF, Snead OC. MEG predicts epileptic zone in lesional extrahippocampal epilepsy: 12 pediatric surgery cases. Epilepsia. 2001. 42:1523–1530.
14. Minassian BA, Otsubo H, Weiss S, Elliott I, Rutka JT, Snead OC 3rd. Magnetoencephalographic localization in pediatric epilepsy surgery: comparison with invasive intracranial electroencephalography. Ann Neurol. 1999. 46:627–633.
15. Knowlton RC, Elgavish RA, Limdi N, Bartolucci A, Ojha B, Blount J, Burneo JG, Ver Hoef L, Paige L, Faught E, et al. Functional imaging: I. Relative predictive value of intracranial electroencephalography. Ann Neurol. 2008. 64:25–34.
16. Sutherling WW, Mamelak AN, Thyerlei D, Maleeva T, Minazad Y, Philpott L, Lopez N. Influence of magnetic source imaging for planning intracranial EEG in epilepsy. Neurology. 2008. 71:990–996.
17. Funke M, Constantino T, Van Orman C, Rodin E. Magnetoencephalography and magnetic source imaging in epilepsy. Clin EEG Neurosci. 2009. 40:271–280.
18. Seo JH, Holland K, Rose D, Rozhkov L, Fujiwara H, Byars A, Arthur T, DeGrauw T, Leach JL, Gelfand MJ, et al. Multimodality imaging in the surgical treatment of children with nonlesional epilepsy. Neurology. 2011. 76:41–48.
19. Bast T, Oezkan O, Rona S, Stippich C, Seitz A, Rupp A, Fauser S, Zentner J, Rating D, Scherg M. EEG and MEG source analysis of single and averaged interictal spikes reveals intrinsic epileptogenicity in focal cortical dysplasia. Epilepsia. 2004. 45:621–631.
20. Iida K, Otsubo H, Matsumoto Y, Ochi A, Oishi M, Holowka S, Pang E, Elliott I, Weiss SK, Chuang SH, et al. Characterizing magnetic spike sources by using magnetoencephalography-guided neuronavigation in epilepsy surgery in pediatric patients. J Neurosurg. 2005. 102:187–196.
21. Oishi M, Kameyama S, Masuda H, Tohyama J, Kanazawa O, Sasagawa M, Otsubo H. Single and multiple clusters of magnetoencephalographic dipoles in neocortical epilepsy: significance in characterizing epileptogenic zone. Epilepsia. 2006. 47:355–364.
22. RamachandranNair R, Otsubo H, Shroff MM, Ochi A, Weiss SK, Rutka JT, Snead OC 3rd. MEG predicts outcome following surgery for intractable epilepsy in children with normal or nonfocal MRI findings. Epilepsia. 2007. 48:149–157.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download