Abstract
Angiogenesis is essential for tumor growth and metastasis. Currently, the Chalkley assay with CD34 immunostaining is the proposed standard method for angiogenesis quantification in solid tumor sections. The purpose of this study was to evaluate the expression of CD34 and its prognostic significance using the Chalkley method in node negative carcinoma of the ampulla of Vater. Between January 1997 and December 2006, 56 node negative patients who had curative resection for carcinoma of the ampulla of Vater were retrospectively reviewed. The Chalkley count was expressed as the mean value of the three counts for each tumor and further divided into two groups according to the mean value of the Chalkley count: low < 4 or high ≥ 4. The mean Chalkley count value was 4.0 (± 3.1). In the low Chalkley group, the 1- and 3-yr recurrence rates were 18.3%, 47.6% respectively; in the high Chalkley group, the 1- and 3-yr recurrence rates were 26.5% and 60.6% respectively. Only high Chalkley count had statistical significance as a factor in recurrence of node negative ampulla of Vater carcinoma. Assessment of angiogenesis may have an important role in the prognostic evaluation of node negative cancer of the ampulla of Vater.
Carcinomas of the ampulla of Vater have a higher resection rate, lower recurrence rate and more favorable prognosis than other malignant tumors of the periampullary region (1, 2). However, tumor recurrences remain frequent problems after surgical treatment of carcinomas of the ampulla of Vater. In ampulla of Vater cancer, the important prognostic factors include the TNM stage, cell differentiation and histologic type. We often encounter a substantial number of patients whose prognosis is not consistent with the TNM stage, and other prognostic criteria must be used in place of the TNM staging system that is widely utilized at present.
Angiogenesis is the development of new vessels from preexisting vessels, and is essential for tumor growth and metastasis (3). The prognostic value of angiogenesis in various types of carcinoma has been widely studied since Weidner et al. (4) reported that high vascular scores were associated with distant metastasis. Currently, the Chalkley assay with CD 34 immunohistostaining is the proposed standard method for angiogenesis quantification in solid tumor sections, according to an international consensus report (5). Many studies have reported that the angiogenesis is a powerful prognostic factor in breast cancer (4, 6, 7). There are no previous studies on angiogenesis in ampulla of Vater carcinoma.
Therefore, the purpose of this study was to evaluate the expression of CD34 and its prognostic significance using the Chalkley method in node negative carcinoma of the ampulla of Vater.
Between January 1997 and December 2006, 96 patients received a radical resection for carcinoma of the ampulla of Vater at Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
Among these 96 patients, 10 were excluded based on the criteria that tissue samples were not well preserved, clinicopathologic data was incomplete or follow-up was lost. As a result, 86 patients who had undergone curative resection were retrospectively reviewed.
Among the 86 patients described above, 56 patients were node negative and 30 were node positive. Ultimately, 56 node negative patients were enrolled in this study. The patients were followed closely until December 31, 2010. All patients were followed up for more than six months.
The 5 µm serial sections of each block were adhered to poly-Llysin covered slides, and incubated at 62℃ for 60 min. After paraffin elimination with xylene and staged ethyl alcohol dehydration, the sections were heated in a microwave containing a 10 mM citrate buffer (pH 6.0) solution for 15 min. The primary antibody used against CD 34 was from clone QBEND/10 (NovoCastra, Newcastle-upon-Tyne, UK). The procedure has been described in detail elsewhere (8).
Angiogenesis in cancer of the ampulla or Vater was evaluated by two pathologists who had no information regarding clinical outcomes. We evaluated tumor vascularization using the Chalkely method as described by Fox et al. (9). Briefly, the CD 34 stained sections were scanned at low magnification for the most vascular area within the tumor section, and three hotspot areas were chosen subjectively. A 25-point Chalkey eyepiece graticule was applied to each hot spot at a higher magnification (X 200 magnification, corresponding to an area of 0.196 mm2), and oriented to permit the maximum number of points to hit on or within the immunohistochemically stained microvessels. The Chalkley count was expressed as the mean value of the three counts for each tumor and further divided into two groups according to the mean value of the Chalkley count: low < 4 or high ≥ 4.
To examine the correlation between the Chalkley count and clinicopathologic variables, we used the chi-square test. For survival analysis, we performed a Kaplan-Meier survival curve based on the log-rank test using the SPSS version 11.0. Overall survival was defined as the time interval between the date of surgery and the date of death from the cancer or last follow-up date. Recurrence-free survival was defined by the time interval between the date of surgery and the date of recurrence or last follow-up date. Multivariate survival analysis was performed using a stepwise forward inclusion algorithm of Cox proportional hazard model. P values of less than 0.05 were considered to be statistically significant.
A total of 56 node negative patients were selected for this study. The mean age was 56.3 yr (± 10.1 yr) and the group was consisted of 31 men and 25 women. All patients underwent pancreaticoduodenectomy (or pylorus preserving pancreaticoduodenectomy) and dissection of the lymph nodes in the hepatoduodenal ligament, the common hepatic artery and the celiac axis.
The mean Chalkley count was 4.0 (± 3.1). Thirty-six of 56 patients were categorized into the low (< 4), and 20 of 56 patients were into the high expression group (≥ 4).
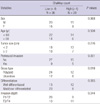
A high Chalkley count was significantly associated with the depth of tumor invasion and perineural invasion. However, the Chalkley count was not significantly correlated with sex, age, tumor size or cell differentiation (Table 1).
For the 56 node negative patients who received radical resection for ampulla of Vater cancer, the 3- and 5-yr overall survival rate were 47.2% and 44.1%, respectively (Fig. 1).
The 1- and 3-yr disease free survival (DFS) was 79.8% and 42.9% respectively (Fig. 2). In univariate analysis, high Chalkley count showed statistical significance factors as DFS in node negative ampulla of Vater carcinoma (Table 2). In multivariate analysis, only high Chalkley count was identified as an independent significance factors as DFS in node negative ampulla of Vater carcinoma (Table 3). In the low Chalkley group, the 1- and 3-yr DFS were 81.7% and 52.4% respectively; in the high Chalkley group, the 1- and 3-yr DFS were 73.5% and 39.4% respectively (Fig. 3).
Angiogenesis is the formation of new vessels from the existing vascular network, and is essential for tumor growth and metastatic capacity. Neovascularization of a tumor is required to provide essential nutrients beyond the limit of simple diffusion, and to allow for tumor growth beyond 2 µL (10). It is well known that the degree of angiogenesis is associated with tumor progression in breast cancer, lung cancer and prostate cancer (7, 11-13). This suggests that angiogenesis is a significant prognostic indicators in patients with carcinoma of the ampulla of Vater. However, the role of angiogenesis in this cancer remains unclear.
The Chalkley count is the number of grid point that hit stained vessels, taken as the average from the assessment of three hot spots. Quantifying angiogenesis using the Chalkley method has been recommended in an international consensus report (5). This method has been thought to have an advantage in that it improves the objectivity of evaluation by eliminating the dependency on an observer in microvessel density measuring. However, an observer is still necessary for the selection of hotspot areas for microvessel quantification.
In the immunohistochemical angiogenesis technique, antibodies to CD 34, CD31 and the von Willebrand factor (factor VIII) have been used to detect vascular structures. Among these antibodies, CD34 was chosen for this study, as it has been shown to give more immunohistochemical staining of microvessels than either CD31 or factor VIII (14). CD34 is a cell surface protein that is selectively expressed by vascular endothelial cells and has been suggested as a standard method for measuring angiogenesis in solid tumor sections in an international consensus report (5).
In this study, angiogenesis was associated only with tumor invasion. Angiogenesis as evaluated with the Chalkley method was not associated with other tumor systems except tumor invasion, which was a finding very similar to many previous ovarian cancer studies using different evaluation method (15, 16). Presumably, our results might have been caused by the following reasons: first, for technical reasons, the difference in the time of ischemic onset due to the time gap between tissue resection and freezing might have affected the immunohistochemical results (17, 18); second, a pathologic diagnosis of ampullar of Vater cancer is usually made during an endoscopic biopsy performed prior to a radical operation. As a result, normal wound healing procedure is initiated and characterized by a substantial neovascular response. These regions might contain a large number of microvessels, and may be mistakenly selected as the designated hotspots. Identification of the regions of neovascularization associated with prior endoscopic biopsy sites must be excluded as potential regions of maximum microvessel density. Therefore, further studies are warranted to examine angiogenesis in a large group of ampulla of Vater cancer patients.
Most studies evaluating the association between tumor progression and angiogenesis have reported their results after dividing the patient population into two groups on the basis of average microvessel count cutoff. Nakayama et al. (19) reported different features of angiogenesis between ovarian and breast carcinoma and found that angiogenesis in ovarian carcinoma was lower than in breast carcinoma as evaluated by intratumoural microvessel density; we found that the median Chalkley count was lower than that of breast cancer. Our data were compatible with previous reports, as increasingly aggressive behavior was shown in node negative cancer of the ampullar of Vater ranging from low to high microvessel counts. A Cox's proportional hazards analysis revealed a statistically significant association between increasing microvessel counts and increased tumor recurrence. However, angiogenesis might not be an all or nothing phenomenon with respect to tumor growth. Progressive increases in aggressive behavior exhibited by tumors may be associated with increasing extents of angiogenesis.
The role of postadjuvant chemotherapy in patients with node negative ampulla of Vater cancer is controversial. We have shown that elevated Chalkley counts (> 4) are related to disease recurrence in patients with node negative tumors. Hence, the group of node-negative patients with Chalkley counts of > 4 is of potential interest for evaluating the effects of systemic adjuvant treatment. Furthermore, the vessel estimate may be suitable for stratification for antiangiogenic treatment.
In conclusion, assessment of angiogenesis may have an important role in the prognostic evaluation of node negative ampulla of Vater cancer. Investigation of the mechanism of angiogenesis in cancer of the ampulla of Vater may provide further prognostic information and help to rationalize therapy. These markers may be useful in selecting patients for chemotherapeutic treatment protocols including the use of antiangiogenic agents.
Figures and Tables
Fig. 3
Disease free survival (DFS) rate in node negative ampulla of Vater carcinoma accrording to Chalkley count.

Table 1
Correlation between angiogenesis and clinicopathological characteristics in ampulla of Vater cancer
References
1. Willett CG, Warshaw AL, Convery K, Compton CC. Patterns of failure after pancreaticoduodenectomy for ampullary carcinoma. Surg Gynecol Obstet. 1993. 176:33–38.
2. Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Lillemoe KD, Pitt HA. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg. 1998. 227:821–831.
3. Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003. 3:401–410.
4. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. N Engl J Med. 1991. 324:1–8.
5. Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, Beliën JA, De Waal RM, Van Marck E, Magnani E, et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002. 38:1564–1579.
6. Bosari S, Lee AK, DeLellis RA, Wiley BD, Heatley GJ, Silverman ML. Microvessel quantitation and prognosis in invasive breast carcinoma. Hum Pathol. 1992. 23:755–761.
7. Horak ER, Leek R, Klenk N, LeJeune S, Smith K, Stuart N, Greenall M, Stepniewska K, Harris AL. Angiogenesis, assessed by platelet/endothelial cell adhesion molecule antibodies, as indicator of node metastases and survival in breast cancer. Lancet. 1992. 340:1120–1124.
8. Hansen S, Grabau DA, Sorensen FB, Bak M, Vach W, Rose C. The prognostic value of angiogenesis by Chalkley counting in a confirmatory study design on 836 breast cancer patients. Clin Cancer Res. 2000. 6:139–146.
9. Fox SB, Leek RD, Smith K, Hollyer J, Greenall M, Harris AL. Tumor angiogenesis in node-negative breast carcinomas-relationship with epidermal growth factor receptor, estrogen receptor, and survival. Breast Cancer Res Treat. 1994. 29:109–116.
10. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990. 82:4–6.
11. Brawer MK, Deering RE, Brown M, Preston SD, Bigler SA. Predictors of pathologic stage in prostatic carcinoma. The role of neovascularity. Cancer. 1994. 73:678–687.
12. Macchiarini P, Fontanini G, Hardin MJ, Squartini F, Angeletti CA. Relation of neovascularisation to metastasis of non-small-cell lung cancer. Lancet. 1992. 340:145–146.
13. Toi M, Kashitani J, Tominaga T. Tumor angiogenesis is an independent prognostic indicator in primary breast carcinoma. Int J Cancer. 1993. 55:371–374.
14. Martin L, Green B, Renshaw C, Lowe D, Rudland P, Leinster SJ, Winstanley J. Examining the technique of angiogenesis assessment in invasive breast cancer. Br J Cancer. 1997. 76:1046–1054.
15. Alvarez AA, Krigman HR, Whitaker RS, Dodge RK, Rodriguez GC. The prognostic significance of angiogenesis in epithelial ovarian carcinoma. Clin Cancer Res. 1999. 5:587–591.
16. van Diest PJ, Zevering JP, Zevering LC, Baak JP. Prognostic value of microvessel quantitation in cisplatin treated FIGO 3 and 4 ovarian cancer patients. Pathol Res Pract. 1995. 191:25–30.
17. Helin HJ, Helle MJ, Kallioniemi OP, Isola JJ. Immunohistochemical determination of estrogen and progesterone receptors in human breast carcinoma. Correlation with histopathology and DNA flow cytometry. Cancer. 1989. 63:1761–1767.
18. Rosenthal LJ. Discrepant estrogen receptor protein levels according to surgical technique. Am J Surg. 1979. 138:680–681.
19. Nakayama K, Kanzaki A, Takebayashi Y, Toi M, Bando H, Nabei T, Miyazaki K, Fukumoto M. Different features of angiogenesis between ovarian and breast carcinoma. Cancer Lett. 2001. 170:161–167.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download