Abstract
The elastin metabolism in systemic sclerosis (SSc) has been known to be abnormal. The authors investigated relationship between the clinical manifestations of systemic sclerosis (SSc) and serum levels of soluble elastin-derived peptide (S-EDP) and anti-elastin antibodies. Serum samples were obtained from 79 patients with SSc and 79 age- and sex-matched healthy controls. Concentrations of serum S-EDP and anti-elastin antibodies were measured by ELISA. The serum concentrations of S-EDP in SSc patients were significantly higher than in healthy controls (median, 144.44 ng/mL vs 79.59 ng/mL, P < 0.001). Serum EDP concentrations were found to be correlated with disease duration in SSc (P = 0.002) and particularly in diffuse cutaneous SSc (P = 0.005). Levels of anti-elastin antibodies were found to be more elevated in SSc patients than in healthy controls (median, 0.222 U vs 0.191 U, P = 0.049), more increased in diffuse cutaneous SSc than limited cutaneous SSc (median, 0.368 U vs 0.204 U, P = 0.031). In addition, levels of anti-elastin antibodies were also found to be negatively associated with presence of anti-centromere antibody (P = 0.023). The S-EDP levels were not found to be correlated with levels of anti-elastin antibodies. The increased S-EDP and anti-elastin antibody levels and association with clinical and laboratory characteristics may reflect the abnormal metabolism in SSc.
Systemic sclerosis (SSc) is a chronic multisystem autoimmune disease with a complex, multifactorial pathogenesis. Several investigators have shown that changes in matrix biology play an important role in the pathogenesis of SSc, which is characterized by an accumulation of collagen in the extracellular matrix by activated fibroblasts (1).
Elastin is an extremely insoluble fibrous protein and a constituent of the extracellular matrix (ECM). It is the main protein of elastic fiber and contributes to the elastic properties of several tissues, such as, the vascular wall, lungs, and skin (2). Furthermore, degradation of elastin by activated proteinases results in the pathologic release of large amounts of soluble elastin-derived peptides (S-EDPs) (3, 4), and elevations of serum S-EDP have been reported in various diseases, such as emphysema, abdominal aortic aneurysm and atherosclerosis (5-7).
In SSc, elastin production is increased in skin (8, 9), and the levels of elastin cross-linking product are increased in urine (10). In addition, previous reports have shown that levels of anti-elastin antibody are elevated in SSc (11, 12). Therefore, we postulated that elastin degradation and S-EDP release into the circulation would be elevated in SSc, and that abnormal elastin metabolism may provoke the production of anti-elastin antibody. In the present study, we analyzed serum levels of S-EDP and of anti-elastin antibody in patients with SSc and searched for associations between these and the clinical manifestations of SSc.
Serum samples were drawn from SSc patients and controls after obtaining informed consent in Seoul National University Hospital from 1998 to 2008. Seventy-nine SSc patients and 79 age- and sex-matched healthy controls were selected. Clinical manifestations were collected by reviewing medical records. The following clinical information was collected: age at enrollment and at disease onset, gender, cutaneous subset, autoantibody status (anti-centromere antibody and anti-Scl-70 antibody), modified Rodnan skin score, and major organ involvement, including the kidneys, heart, lungs, and pulmonary artery. Cutaneous subsets were classified as diffuse or limited based on the classification by LeRoy et al. (13) with some modification; the limited cutaneous subset was defined as those with scleroderma limited to the hands, face, feet and forearms. Major organ involvements were classified as follows. Interstitial lung disease was defined as the presence of bibasilar pulmonary fibrosis by chest radiography or high-resolution computed tomography without any evidence of other lung disease. Heart involvement was defined as the presence of major conduction disturbance, ventricular arrhythmia, heart failure, or persistent pericardial effusion for ≥ 2 months. Pulmonary hypertension was defined as a systolic pulmonary artery pressure of > 35 mmHg by echocardiography. The number of patients with renal involvement was only 1, and thus, renal involvement was excluded from the analysis. Disease duration was calculated from the appearance of the first symptoms besides Raynaud' phenomenon.
S-EDP concentration was determined by competitive ELISA adapted from a protocol described by Sivaprasad et al. (14). Soluble α-elastin was obtained from human aorta (Sigma-Aldrich, Poole, UK), and the rabbit anti-human elastin IgG (Elastin Products Co., Owensville, MO, USA) was raised against α-elastin prepared from human aorta.
Briefly, the wells of a microtiter plate (Nunc, Fischer Scientific, Loughborough, UK) were coated with 150 µL α-elastin (1 µg/mL) in 0.1 M sodium carbonate (pH 9) by incubating at room temperature for 2 hr. The plate was then washed three times with phosphate-buffered saline (PBS) containing 0.05% (v/v) Tween 20 (PBST). A curve was generated using dilutions of human aortic α-elastin (0-1 µg/mL). Anti-α-elastin antibodies (1:3,000) in PBST and 3% (w/v) bovine serum albumin (PBST-3% BSA) were preincubated with variable dilutions of α-elastin in PBST-3% BSA for 1 hr 30 min at 37℃. These solutions were then transferred to the coated plate and incubated at 37℃ for 1 hr. The preincubation period before adding the solution to α-elastin-coated microtiter plate creates the competitive aspect of the ELISA. Before adding detection antibody to wells, the microtiter plate wells were washed with PBST three times. Then, horse radish peroxidase (HRP) conjugated goat anti-rabbit IgG antibody (Santa Cruz Biotechnology/Autogen Bioclear, Santa Cruz, CA, USA) was added to each well. The plate was incubated for 20 min at room temperature and again washed with PBST three times. Thereafter, 2,2'-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS) (Calbiochem, Darmstadt, Germany) buffer was added to each well, and the plate was incubated for up to 30 min at room temperature. Absorbance was read at 405 nm using an automated plate reader (Molecular Devices, Sunnyvale, CA, USA). A standard curve was generated from absorbance readings obtained from α-elastin dilutions. Standards were analyzed in triplicate, and serum samples were analyzed in duplicate. S-EDP concentrations were calculated from the standard curve and are expressed as nanograms per milliliter.
Anti-human elastin antibody assays were performed using a modified ELISA protocol (15). Briefly, human skin elastin peptides were purchased from Elastin Products Co. (Owensville, MO, USA) in lyophilized form and dissolved in water to a final concentration of 25 µg/mL, and 40 µL aliquots of this working solution were used to coat high protein binding flat bottomed polystyrene microtiter plates (Immulon 4HBX, Fisher Scientific Company, Pittsburgh, PA, USA). The plates were then incubated at 37℃ for 2 hr or alternatively at 4℃ overnight. After washing with PBST, plates were blocked by adding 0.2% I-block (Tropix, Bedford, MA, USA) and incubated at 37℃ for 2 hr. After washing, human serum samples were diluted in 100 mM sodium bicarbonate buffer (pH 9.4) in polypropylene microtiter plates and incubated at 37℃ for 2 hr. After washing, biotinylated chicken anti-human IgG antibody was added and plates were incubated at 37℃ for 2 hr. Plates were washed, alkaline phosphatase conjugated streptavidin was added, and plates were incubated at room temperature for an additional 30 min. After a final wash, nitrophenyl phosphate substrate was added. The colorimetric reaction was terminated by adding 0.5 N sodium hydroxide per well and the optical densities of individual wells were determined at 405 nm using a standard microplate spectrophotometer (Wallac Victor 2, Perkin Elmer, Waltham, MA, USA).
Serum levels of S-EDP and anti-elastin antibody in SSc patients and healthy controls were compared using the Mann-Whitney U test. The levels of S-EDP and anti-elastin antibody in SSc patients and in healthy controls were analyzed using the Mann-Whitney U test and analysis of covariance. Associations between continuous variables and S-EDP or anti-elastin antibody levels were analyzed using Spearman's correlation analysis. All statistical analyses were two-tailed and P values of < 0.05 were considered significant. Statistical analysis was performed using SPSS version 12.0K.
Of the 79 patients, 75 (95%) were females; 37 patients had the limited cutaneous subtype and 42 patients the diffuse cutaneous subtype. Mean age (± SD) at diagnosis was 49.7 ± 12.2 yr and disease duration (mean ± SD) was 9.3 ± 7.3 yr. Anti-centromere antibody was found in 11 patients and anti-Scl-70 antibody in 51 patients. Modified Rodnan skin scores were available for 47 (59.5%) patients. Eight patients had heart involvement, 5 had pulmonary arterial hypertension, and 59 had lung involvement. Only one patient had kidney involvement, and thus, kidney involvement was not analyzed as a variable.
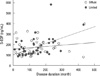
The serum concentrations of S-EDP in SSc patients (median [range], 144.44 ng/mL [80.06, 583.25]) were significantly higher than in healthy controls (median [range], 79.59 ng/mL [45.75, 180.55]) as shown in Fig. 1 (P < 0.001, by Mann-Whitney U test). Age was positively correlated with S-EDP level in SSc patients (Spearman's ρ = 0.245, P = 0.029), but negatively correlated in controls (Spearman's ρ = -0.322, P = 0.003, by Spearman's correlation analysis). In addition, S-EDP levels were found to be correlated with disease duration in SSc (Spearman's ρ = 0.346, P = 0.002, by Spearman's correlation analysis), and especially, in the diffuse cutaneous subtype (diffuse: Spearman's ρ = 0.429, P = 0.005; limited: Spearman's ρ = 0.212, P = 0.214, by Spearman's correlation analysis, Fig. 2). Serum levels of S-EDP were not different between patients with limited SSc and diffuse SSc (P = 0.829, by Mann-Whitney U test) (Table 1). Furthermore, S-EDP levels were not found to be associated with the presence of anti-centromere antibody (P = 0.892), anti-Scl70 antibody (P = 0.581), interstitial lung disease (P = 0.660), heart involvement (P = 0.667), or pulmonary hypertension (P = 0.163, all P values by Mann-Whitney U test). The correlation between S-EDP levels and modified Rodnan skin scores was not significant in all patients, or in patients with the diffuse cutaneous or limited cutaneous subtypes (total, P = 0.280; diffuse, P = 0.176; limited, P = 0.990, by Spearman's correlation analysis). After adjusting for disease duration, S-EDP levels were not found to be dependent on the presence of clinical features, anti-Scl70 antibody or anti-centromere antibody.
The serum concentrations of anti-elastin antibodies were elevated in SSc patients versus controls (median [range], SSc: 0.222 U [0.050, 3.548], controls: 0.191 U [0.004, 3.796], P = 0.049, by Mann-Whitney U test). Neither age nor disease duration was correlated with anti-elastin antibody in SSc patients (age, P = 0.267; disease duration, P = 0.363, by Spearman's correlation analysis). However, anti-elastin antibody levels were significantly higher in the diffuse cutaneous subtype than in the limited cutaneous subtype (P = 0.031, by Mann-Whitney U test), and anti-elastin antibody levels were found to be higher in the absence of anti-centromere antibody (P = 0.023, by Mann-Whitney U test), but anti-elastin antibody levels was not found to be correlated with modified Rodnan skin scores or any other clinical organ involvements (Table 2). S-EDP and anti-elastin antibody levels were not correlated in SSc patients (P = 0.628), and in the diffuse (P = 0.575) and limited cutaneous subtypes (P = 0.929).
This study showed that serum levels of S-EDP and anti-elastin antibody were elevated in SSc patients versus healthy age- and sex-matched control. Moreover, a positive association was found between S-EDP levels and disease duration, which supports the suggestion that EDP is closely related to disease process. Furthermore, anti-elastin antibody level was found to be increased in diffuse cutaneous SSc and in the absence of anti-centromere antibody.
The reason why the level of S-EDP increased in SSc is not clear. The correlations between S-EDP levels and disease duration or age might provide clues to the reason for this phenomenon. In previous morphologic studies, the aging process was found to cause increase in elastic fiber (16) and elastic fiber had changes suggestive of degradation in the normal skin age-dependently (8). However, in this study, age was found to be inversely correlated with S-EDP levels, in healthy controls. This discrepancy could be attributed to the limitation in morphologic study. Thus, the increased S-EDP level found in SSc cannot be attributed to the aging process, but rather to disease duration, which means that S-EDP is probably associated with the pathologic process. Previously, in a small-scale study, disease duration was not found to be associated with elastin in the skin (9), but in a morphologic analysis, elastic fiber was found to be increased in SSc skin as compared with normal skin (8). Abundant elastin in the skin can be abnormal (17) and actively degraded by adjacent cells (18), which may be the cause of an increase in serum S-EDP in SSc patients. In a previous study, it was found that desmosine and isodesmosine, cross-link amino acids from elastin, increased in the urine of SSc patients, which supports our results (10).
Elastin is known to be present in blood vessels and lung tissues, which can also be sources of EDP, which is produced by several enzymes, including elastase from leukocytes. Furthermore, several authors have suggested that active inflammation in SSc can augment the degradation of elastin. Desmosine and isodesmosine released during elastin degradation have been reported to be negatively associated with the carbon monoxide diffusion capacity of the lung in SSc (10) and interstitial lung disease in SSc has been associated with the levels of serum or bronchoalveolar lavage fluid neutrophil elastase (19, 20). Pulmonary hypertension has also been associated with elastin production and degradation (21, 22). In the present study, S-EDP levels were not found to be related to clinical subtypes, modified Rodnan skin scores, or organ involvement despite a relatively large cohort size and the enrollment of age-sex matched controls. It may be that the systemic nature of SSc and the time between elastin synthesis and degradation obscure differences in clinical subtypes or organ involvement (including skin thickness).
EDP is known to have many different biologic effects, for example, it can recruit fibroblasts, endothelial cells, and monocytes like chemokines (23-25), and also stimulates arterial smooth muscle cell proliferation (26) and Th1 differentiation (27). The precise role played by EDP in SSc is not known, but in view of the effect that EDP has on different cells, it appears to be a candidate for involvement in the pathogenesis in SSc. Further functional studies on EDP are required to explore its role in SSc.
Previously, the presence of anti-elastin antibody was considered to reflect elastin degradation (28). However, in the present study, levels of EDP, the direct product of elastin degradation, were not found to be correlated with anti-elastin antibody levels. Serum anti-elastin antibody levels are known to increase in various connective tissue diseases (12) and humoral and cell-mediated immune response against elastin in SSc patients has been demonstrated (11), which suggests that anti-elastin antibody production is as a result of a secondary response to increased elastin production or degradation. The association of anti-elastin antibody with diffuse cutaneous subtype or negativity of anti-centromere antibody may reflect different immunologic profiles in each subtype.
In summary, serum levels of S-EDP and anti-elastin antibody are elevated in SSc patients versus healthy age- and sex-matched control. The serum level of S-EDP was associated with disease duration. Moreover, anti-elastin antibody level was increased in diffuse cutaneous SSc and in the absence of anti-centromere antibody.
Figures and Tables
Fig. 1
Serum concentrations of S-EDP in SSc patients. Serum concentrations of S-EDP in SSc patients were significantly higher than those in healthy controls (P < 0.001, by Mann-Whitney U test). The thick horizontal lines in boxes are medians and the upper and lower sides of the boxes represent upper and lower quartiles, respectively. The horizontal bars above and below each box represent highest and lowest values within the 1.5 times of interquartile range and circles represent outliers.

Fig. 2
The levels of S-EDP and disease durations in systemic sclerosis. The levels of S-EDP and disease durations were correlated in systemic sclerosis (Spearman's ρ = 0.346, P = 0.002) and especially diffuse cutaneous subtype (Spearman's ρ = 0.429, P = 0.005).
References
1. Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009. 360:1989–2003.
2. Debelle L, Tamburro AM. Elastin: molecular description and function. Int J Biochem Cell Biol. 1999. 31:261–272.
3. Bizbiz L, Alpérovitch A, Robert L. Aging of the vascular wall: serum concentration of elastin peptides and elastase inhibitors in relation to cardiovascular risk factors. The EVA study. Atherosclerosis. 1997. 131:73–78.
4. Fülöp T Jr, Wei SM, Robert L, Jacob MP. Determination of elastin peptides in normal and arteriosclerotic human sera by ELISA. Clin Physiol Biochem. 1990. 8:273–282.
5. Pardo A, Selman M. Proteinase-antiproteinase imbalance in the pathogenesis of emphysema: the role of metalloproteinases in lung damage. Histol Histopathol. 1999. 14:227–233.
6. Lindholt JS, Heickendorff L, Henneberg EW, Fasting H. Serum-elastin-peptides as a predictor of expansion of small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 1997. 14:12–16.
7. Petersen E, Wågberg F, Anquist KA. Serum concentrations of elastin-derived peptides in patients with specific manifestations of atherosclerotic disease. Eur J Vasc Endovasc Surg. 2002. 24:440–444.
8. Quaglino D Jr, Bergamini G, Boraldi E, Manzini E, Davidson J, Pasquali Ronchetti I. Connective tissue in skin biopsies from patients suffering systemic sclerosis. J Submicrosc Cytol Pathol. 1996. 28:287–296.
9. Pasquali Ronchetti I, Guerra D, Quaglino D Jr, Vincenzi D, Manzini E, Canossi B, Manzini CU. Dermal elastin and collagen in systemic sclerosis. Effect of D-penicillamine treatment. Clin Exp Rheumatol. 1989. 7:373–383.
10. Stone PJ, Korn JH, North H, Lally EV, Miller LC, Tucker LB, Strongwater S, Snider GL, Franzblau C. Cross-linked elastin and collagen degradation products in the urine of patients with scleroderma. Arthritis Rheum. 1995. 38:517–524.
11. Daskalova M, Taskov H, Dimitrova E, Baydanoff S. Humoral and cellular immune response to elastin in patients with systemic sclerosis. Autoimmunity. 1997. 25:233–241.
12. Colburn KK, Langga-Shariffi E, Kelly GT, Malto MC, Sandberg LB, Baydanoff S, Green LM. Abnormalities of serum antielastin antibodies in connective tissue diseases. J Investig Med. 2003. 51:104–109.
13. LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, Rowell N, Wollheim F. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988. 15:202–205.
14. Sivaprasad S, Chong NV, Bailey TA. Serum elastin-derived peptides in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2005. 46:3046–3051.
15. Lee SH, Goswani S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007. 13:567–569.
16. Pasquali-Ronchetti I, Baccarani-Contri M. Elastic fiber during development and aging. Microsc Res Tech. 1997. 38:428–435.
17. Rustin MH, Papadaki L, Rode J, Dowd PM. Elastic fibers in patients with systemic sclerosis. A morphological study. Virchows Arch A Pathol Anat Histopathol. 1989. 416:115–120.
18. Davis EC, Blattel SA, Mecham RP. Remodeling of elastic fiber components in scleroderma skin. Connect Tissue Res. 1999. 40:113–121.
19. Crestani B, Seta N, Palazzo E, Rolland C, Venembre P, Dehoux M, Boutten A, Soler P, Dombret MC, Kahn MF, et al. Interleukin-8 and neutrophils in systemic sclerosis with lung involvement. Am J Respir Crit Care Med. 1994. 150:1363–1367.
20. Hara T, Ogawa F, Yanaba K, Iwata Y, Muroi E, Komura K, Takenaka M, Shimizu K, Hasegawa M, Fujimoto M, et al. Elevated serum concentration is of polymorphonuclear neutrophilic leukocyte elastase in systemic sclerosis: association with pulmonary fibrosis. J Rheumatol. 2009. 36:99–105.
21. Rabinovitch M. Elastase, remodeling of extracellular matrix, and pulmonary hypertension. Semin Respir Crit Care Med. 1994. 15:199–206.
22. Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004. 43:13S–24S.
23. Senior RM, Griffin GL, Mecham RP. Chemotactic responses of fibroblasts to tropoelastin and elastin-derived peptides. J Clin Invest. 1982. 70:614–618.
24. Hance KA, Tataria M, Ziporin SJ, Lee JK, Thompson RW. Monocytic chemotactic activity in human abdominal aortic aneurysms: role of elastin degradation peptides and the 67-kD cell surface elastin receptor. J Vasc Surg. 2002. 35:254–261.
25. Robinet A, Fahem A, Cauchard JH, Huet E, Vincent L, Lorimier S, Antonicelli F, Soria C, Crepin M, Hornebeck W, et al. Elastin-derived peptides enhance angiogenesis by promoting endothelial cell migration and tubulogenesis through upregulation of MT1-MMP. J Cell Sci. 2005. 118:343–356.
26. Mochizuki S, Brassart B, Hinek A. Signaling pathways transduced through the elastin receptor facilitate proliferation of arterial smooth muscle cells. J Biol Chem. 2002. 277:44854–44863.
27. Debret R, Antonicelli F, Theill A, Hornebeck W, Bernard P, Guenounou M, Le Naour R. Elastin-derived peptides induced a T-helper type 1 polarization of human blood lymphocytes. Arterioscler Thromb Vasc Biol. 2005. 25:1353–1358.
28. Colburn KK, Kelly GT, Malto MC, Sandberg LB, Boucek RJ. Serum anti-tropo: anti-alpha-elastin antibody ratio assessing elastin turnover in scleroderma. Clin Rheumatol. 1992. 11:206–210.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download