Abstract
Neurologic complications of children with influenza A H1N1 2009 pandemic, diagnosed in two consecutive influenza seasons were retrospectively reviewed to seek better outcomes in future outbreaks. Patient demographics, clinical manifestations and neurologic outcomes were reviewed. A total of 1,389 children were diagnosed with influenza A H1N1 by real-time reverse transcriptase-polymerase chain reaction. Of these, 23 (1.7%) patients had neurologic involvement. Their mean age was 5.9 ± 3.6 yr (range, 6 months to 11 yr) and 16 (69.9%) were boys. None of the 23 patients had been vaccinated for influenza A H1N1 and seasonal influenzas. Twenty-two of the 23 patients presented with seizures. Clinical features included febrile convulsion (n = 19), afebrile convulsion (n = 1), aseptic meningitis (n = 1), encephalopathy (n = 1), and acute necrotizing encephalopathy (n = 1). They all were treated with Oseltamivir twice daily for 5 days immediately after nasal and throat swab testing. Twenty-one of the subjects recovered fully, but the youngest two infants experienced severe neurological sequelae. The results indicate that neurologic complications associated with influenza A H1N1 2009 pandemic were mostly mild, but rarely were serious. Prompt intervention leads to a better outcome and vaccination may prevent the disease, thus staving off serious neurological complications following influenza, especially in young infants.
The pandemic (H1N1) 2009 caused by a novel influenza A H1N1 virus (hereafter abbreviated as H1N1), which originated in the Mexican town of La Gloria, Veracruz, in February of that year, spread rapidly in Korea after being introduced in the country in April, 2009. The World Health Organization (WHO) declared the pandemic over on August 10, 2010. At that time, the death toll exceeded 18,000 (1). During the pandemic, on November 3, 2009, the Korean government raised the national disaster phase from "alert" to "severe (red)" and the Korean Center for Disease Control and Prevention (KCDC) reported peak H1N1 outbreak from November 15 to December 5, 2009. During this time, approximately 800,000 Koreans were infected and 252 people died due to the H1N1 (2).
In the aftermath of the pandemic (H1N1) 2009, much attention has been focused on the respiratory manifestations of H1N1 (3, 4). Influenza is a major cause of acute respiratory tract illnesses each winter. Influenza virus infection also causes extrapulmonary symptoms that include gastrointestinal or neurological symptoms. However, much less is known regarding neurologic complications. Seasonal influenza A and B viruses have varying degrees of neurovirulence (5) and can cause a variety of neurologic complications including Reye syndrome, Guillian-Barre syndrome, transverse myelitis, encephalopathy, aseptic meningitis, acute disseminated encephalomyelitis (ADEM), acute necrotizing encephalopathy (ANE) and seizures (6, 7). Seizures are the most frequently reported neurologic complication and, in young patients, most of these manifest as febrile convulsion (8). Several investigators reported frequent and severe encephalopathy related to seasonal influenza in the past decade (9, 10). Several studies have suggested that the pandemic (H1N1) 2009 also causes influenza encephalopathy in children (1, 8) and is associated with a notable predominance of disease burden and mortality in children (11).
It is a descriptive analysis of clinical findings from a single institution. We reviewed the demographic characteristics, clinical features and severity of disease of affected patients focusing on neurologic complications for two consecutive cold seasons from September 2009 to March 2011. The goal was to seek management strategies that yield a better neurologic outcome.
Twenty-three Korean children diagnosed with pandemic (H1N1) 2009 were retrospectively evaluated for neurological complications at the Department of Pediatrics in Kyungpook National University Hospital, Daegu, Korea, during the influenza seasons 2009-2010, and 2010-2011. Diagnoses were confirmed by nasal and throat swabs with the use of real-time reverse transcriptionpolymerase chain reaction (RT-PCR) assay.
We reviewed the laboratory surveillance data of our hospital and 1,389 cases were identified among 3,421 patients treated in the pediatric department using virology laboratory records that indicated positive findings for influenza A H1N1. The medical records of the identified patients were reviewed for evidence of neurologic complications, including febrile or non febrile seizures, encephalopathy or altered mental status, meningitis and other neurologic complications.
The aforementioned cases of laboratory confirmed H1N1 were included. Children with an abnormal neurodevelopmental background and who did not exhibit any change from their baseline neurologic condition or were defined as disease of central nervous system were excluded.
A detailed chart review was performed for the 23 patients who had neurologic complications. Data on several clinical variables were retrieved, including age, gender, birth history, developmental history, family history, medical background, chief neurologic complications, laboratory (blood and cerebrospinal fluid) findings, neuroimaging and electroencephalographic findings, treatment and clinical outcomes. We classified the patients according to the neurologic outcome as excellent, good, fair or poor.
The following outcome definitions were used. In an excellent outcome, patients showed complete resolution. In a good outcome, patients showed almost complete resolution or minimal degree of neurological sequelae (mobile, almost no cognitive/social/emotional impairment). In a fair outcome, patients showed moderate degree of neurological sequelae (mobile with difficulty, mild to moderate cognitive/social/emotional impairment). In a poor outcome, patients showed severe degree of neurological sequelae (immobile, severe cognitive/social/emotional impairment). The definition of lymphopenia in this study was a lymphocyte count less than 13 percent of the total number of leukocytes.
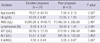
From September 2009 to March 2010, 3,019 Korean children aged 6 months to 11 yr were PCR-tested for H1N1. Of these, 1,326 were positive. From September 2010 to March 2011, 63 patients were positive among 402 children tested. A total of 1,389 children were confirmed with H1N1. Of these, 23 (1.7%) of them had neurological involvement (Fig. 1). H1N1-related neurologic complications noted in 23 patients included febrile convulsion (n = 19), afebrile convulsion (n = 1), aseptic meningitis (n = 1), encephalopathy (n = 1) and acute necrotizing encephalopathy (n = 1). Their mean age of the 16 males and seven females was 5.9 ± 3.6 yr (6 months to 11 yr, range). The mean age of febrile convulsion was 6.4 ± 3.3 yr (1.9 to 11 yr, range), while the age for afebrile convulsion, aseptic meningitis, acute necrotizing encephalopathy and encephalopathy was 2.3 yr, 9.7 yr, 9 months, and 6 months, respectively (Table 1). None of the patients had been vaccinated for H1N1 or seasonal influenza.
Of the 23 patients, 22 presented with seizures (20 febrile convulsion, two afebrile convulsion). Six children experienced status epilepticus and one suffered from refractory status epilepticus. Among the 20 patients with febrile convulsion, nine had simple febrile convulsion and the other 11 patients were > 5 yr of age. Four children previously experienced febrile convulsion and 13 patients displayed respiratory symptoms such as cough, rhinorrhea or sore throat.
Blood laboratory results were basically unremarkable, but 13 patients showed lymphopenia and one patient displayed elevated serum aminotransferase. Cerebrospinal fluid analysis was performed in 14 children; only one showed pleocytosis. This patient was confirmed with aseptic meningitis and recovered fully. Neuroimaging by brain magnetic resonance imaging (MRI) or computed tomography (CT) was done in 10 patients. Of these, two had abnormal findings in the corpus callosum or both thalami. The former was diagnosed with febrile convulsion and fully recuperated without neurologic deficit, but the latter was diagnosed with acute necrotizing encephalopathy and had a poor neurologic outcome. An electroencephalogram was done in 18 patients. The results from three patients were abnormal. Two patients who displayed epileptiform discharges were diagnosed with febrile convulsion and recovered fully after 6 months of onset without following seizures. The other patient who had a diffuse, marked suppression of background activities was diagnosed with encephalopathy and died after 4 months of mechanical ventilation (Table 2).
All patients were treated with Oseltamivir twice daily for 5 days immediately after nasal and throat swab testing. Eleven children additionally received lorazepam, phenytoin, phenobarbital or midazolam continuous drip for seizures. Twenty one patients fully recovered and were discharged. The youngest two patients experienced severe neurological sequelae and one of them died.
We classified the patients according to the neurologic outcome (excellent, good, fair or poor). When compared between the group of excellent and poor outcomes, there were statistically significant differences in age, blood potassium, percentages of polymorphonuclear leukocytes and lymphocytes and in the hemoglobin level (Table 3).
Two cases that presented poor neurologic outcome are described here in more detail. In the youngest case, a 6-monthold male infant had a flu-like illness and febrile status epilepticus. Brain MRI revealed increased signal density on T2-weighted imaging in the bilateral thalami and subcortical white matter (Fig. 2A). The findings were compatible with acute necrotizing encephalopathy. The patient was positive for H1N1 and was treated with oral Oseltamivir, solumedrol pulse therapy and intravenous immunoglobulin (1 g/kg/day) for 2 days. Follow-up MRI showed increased signal density on T2-weighted imaging at the same area (Fig. 2B). The patient was left with moderate degree neurologic sequelae showing quadriparesis with cognitive impairment.
In the other case, a 9-month-old female infant presented with a 2-day history of cough, prolonged afebrile generalized tonic-clonic seizures and respiratory arrest. Chest X-ray and CT revealed bilateral pneumonic consolidation. The patient was also positive for H1N1 and was treated with oral oseltamivir, intravenous cefotaxime, ampicillin and dexamethasone for presumed encephalopathy. The patient remained comatose, required mechanical ventilation for 4 months and ultimately died.
The present study was conducted to evaluate the clinical features in Korean children aged 6 months to 11 yr who visited a single institution during influenza seasons 2009-2010, and 2010-2011.
In Korea, much attention has been paid to H1N1 in the aftermath of the 2009 illness outbreak (12, 13), but little is known regarding H1N1-related neurologic complications in children. A few studies in Western countries have reported a wide spectrum of neurologic manifestations in children associated with the pandemic (H1N1) 2009, ranging from simple febrile convulsion to encephalopathy (14, 15). The reported prevalence of H1N1-related neurological complications in children has been variable, ranging from 2%-15% (14, 16). In this study, 1,389 patients diagnosed with H1N1 by nasal and throat swabs with the use of real-time RT-PCR assay were enrolled. Of these subjects, 23 children (1.7%) had neurologic symptoms. All were hospitalized and two went to pediatric intensive care unit for seizure control and mechanical ventilation.
Respiratory symptoms are the main clinical manifestations of influenza, but extrapulmonary symptoms can also occur. Seizure has been reported as the most common neurologic complication of seasonal influenza A and B (6, 17), as well as pandemic (H1N1) 2009 (8, 18), but shows good prognosis. In contrast, encephalopathy is rare, but it can cause a serious outcome, especially in patients of young age or with underlying neuromuscular disorder (6). In the present study, consistent with other studies, seizure was the most common neurologic complication, occurring in 22 of the 23 children displaying neurologic symptoms. Twenty children had febrile seizures; nine of them were older than 5 yr of age, these nine patients were not considered as high risk for febrile convulsions. Influenza-induced febrile or non-febrile seizures might be associated with the development of epilepsy (19). However, further evaluation and longterm follow-up is required to confirm this relationship. Compared with seasonal influenza, H1N1 is not more severe, but neurologic complications such as encephalopathy, focal neurologic sign, aphasia and abnormal electroencephalogram findings can be more common (11, 20). Neurologic complications resulting from H1N1 in pediatric populations include various clinical manifestations such as seizure, aseptic meningitis, encephalitis and acute disseminated encephalomyelitis and can result in significant acute and residual neurologic sequelae (14, 18). During influenza season, pediatricians should consider influenza as the cause of unexplained mental status change, especially in a patient with an underlying chronic illness, because of more aggressive clinical course. The incidence of neurologic complications are more common in patients who require intensive care (16%-20%), with common symptoms being seizure (6%), encephalopathy (12%), encephalitis (1%) and myositis (1%) (21).
Neurologic complications in this study included febrile or afebrile convulsion, aseptic meningitis and encephalopathy. The outcome of these neurologic complications ranged from spontaneous resolution without sequelae to encephalopathy. With respect to prognosis, a younger age could prelude a poorer outcome; the two youngest patients had moderate to severe sequelae after H1N1 infection. However, immediate and thorough evaluation and aggressive management often produced a good clinical outcome.
Although some insist age does not affect the clinical result (22), it is generally accepted that younger individuals are more vulnerable to severe influenza; this supposition is supported by the present results. Influenza vaccination for infants over 6 months of age could be prudent in reducing severe infections (23). For seasonal influenza, underlying medical conditions such as chronic lung disease, heart disease, immunodeficiency, metabolic disease and specific age group (> 64 yr of age, < 2 yr of age) are risk factors for severe influenza (11). Patients with these specific medical conditions have higher rates of hospitalization and death (24, 25). Also presently, no patient had high risk factors for severe influenza, with the exception of young age (< 2 yr of age). Lymphopenia has been implicated as a major risk factor (26); however, this was not presently apparent. It seemed that a thorough investigation might be helpful in planning treatment strategy and predicting the clinical course. However, this assumption remains unsubstantiated because of the limited number of subjects, especially the group of poor prognosis, in the present study.
In the influenza season from 2010 to 2011, the number of patients suspected of having H1N1 and who were required to get a PCR test was markedly decreased, probably due to improved herd immunity from the previous symptomatic or asymptomatic infections and vaccinations (23). As a result, the incidence of neurologic complications was markedly decreased, consistent with a previous report (27).
In conclusion, neurologic complications from the pandemic (H1N1) 2009 are usually mild and benign, but rarely result in poor neurological sequlae. Early prompt intervention leads to a better outcome and vaccination may prevent the disease, thus staving off serious neurological complications following influenza, especially in young infants.
Although this study provides useful information about neurologic complications associated with H1N1, it has several limitations. We did not perform comparison between the group with pandemic (H1N1) 2009 and the group with seasonal influenza. In addition, this study reports from only single institution in Korea, and the incidence and spectrum of disease could have potential selection bias.
Figures and Tables
Fig. 1
Incidence and percentage of influenza A H1N1 PCR and neurologic complications. During the 2009-2010 influenza season, 3,019 children were PCR-tested for influenza A H1N1. Of these, 1,326 (43.9%) were positive and 20 patients (1.5%) had neurologic complications. From September 2010 to March 2011, 63 patients (15.7%) were positive among 402 children and three patients (4.7%) experienced neurologic complications. A total of 1,389 children were confirmed with influenza A H1N1; 23 (1.7%) of them had neurological involvement.

Fig. 2
Brain MRI of the patient with acute necrotizing encephalopathy. (A) Initial T2-weighted image shows a high signal intensity lesion (arrows) in both thalami. (B) Follow-up brain MRI reveals bilateral symmetric hyperintense lesions (arrows) in the both thalami on T2-weighted image.
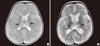
Table 1
Demographic and clinical characteristics of 23 patients with neurological complications due to the pandemic (H1N1) 2009

References
1. Yildizdaş D, Kendirli T, Arslanköylü AE, Horoz OO, Incecik F, Inçe E, Ciftci E. Neurological complications of pandemic influenza (H1N1) in children. Eur J Pediatr. 2011. 170:779–788.
2. Chung DR. Evaluation of the correspondence against Pandemic influenza (H1N1 2009) through the infectious diseases specialist survey. Infect Chemother. 2010. 42:87–89.
3. Jaber S, Conseil M, Coisel Y, Jung B, Chanques G. ARDS and influenza A (H1N1): patients' characteristics and management in intensive care unit. A literature review. Ann Fr Anesth Reanim. 2010. 29:117–125.
4. Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, Jasuja S, Deutscher M, Chen S, Walker JD, Duchin JS, Lett S, Soliva S, Wells EV, Swerdlow D, Uyeki TM, Fiore AE, Olsen SJ, Fry AE, Bridges CB, Finelli L. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009. 361:1935–1944.
5. Oxford JS. Influenza A pandemics of the 20th century with special reference to 1918: virology, pathology and epidemiology. Rev Med Virol. 2000. 10:119–133.
6. Newland JG, Laurich VM, Rosenquist AW, Heydon K, Licht DJ, Keren R, Zaoutis TE, Watson B, Hodinka RL, Coffin SE. Neurologic complications in children hospitalized with influenza: characteristics, incidence, and risk factors. J Pediatr. 2007. 150:306–310.
7. Glezen WP. Feigin RD, Cherry JD, Demmler GJ, Kaplan SL, editors. Influenza viruses. Textbook of pediatric infectious diseases. 2004. 5th ed. Philadelphia: Saunders;2252–2269.
8. Landau YE, Grisaru-Soen G, Reif S, Fattal-Valevski A. Pediatric neurologic complications associated with influenza A H1N1. Pediatr Neurol. 2011. 44:47–51.
9. Morishima T, Togashi T, Yokota S, Okuno Y, Miyazaki C, Tashiro M, Okabe N. Collaborative Study Group on Influenza-Associated Encephalopathy in Japan. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis. 2002. 35:512–517.
10. Togashi T, Matsuzono Y, Narita M, Morishima T. Influenza-associated acute encephalopathy in Japanese children in 1994-2002. Virus Res. 2004. 103:75–78.
11. O'Riordan S, Barton M, Yau Y, Read SE, Allen U, Tran D. Risk factors and outcomes among children admitted to hospital with pandemic H1N1 influenza. CMAJ. 2010. 182:39–44.
12. Kim HS, Kim JH, Shin SY, Kang YA, Lee HG, Kim JS, Lee JK, Cho B. Fatal cases of 2009 pandemic influenza A (H1N1) in Korea. J Korean Med Sci. 2010. 26:22–27.
13. Cho WH, Kim YS, Jeon DS, Kim JE, Kim KI, Seol HY, Kim KU, Park HK, Lee MK, Park SK, et al. Outcome of pandemic H1N1 pneumonia: clinical and radiological findings for severity assessment. Korean J Intern Med. 2011. 26:160–167.
14. Baltagi SA, Shoykhet M, Felmet K, Kochanek PM, Bell MJ. Neurological sequelae of 2009 influenza A (H1N1) in children: a case series observed during a pandemic. Pediatr Crit Care Med. 2010. 11:179–184.
15. Bustos B R, Andrade YF. Acute encephalopathy and brain death in a child with influenza A (H1N1) during the 2009 pandemic. Rev Chilena Infectol. 2010. 27:413–416.
16. Centers for Disease Control and Prevention (CDC). Neurologic complications associated with novel influenza A (H1N1) virus infection in children: Dallas, Texas, May 2009. MMWR Morb Mortal Wkly Rep. 2009. 58:773–778.
17. Maricich SM, Neul JL, Lotze TE, Cazacu AC, Uyeki TM, Demmler GJ, Clark GD. Neurologic complications associated with influenza A in children during the 2003-2004 influenza season in Houston, Texas. Pediatrics. 2004. 114:e626–e633.
18. Surana P, Tang S, McDougall M, Tong CY, Menson E, Lim M. Neurological complications of pandemic influenza A H1N1 2009 infection: European case series and review. Eur J Pediatr. 2011. 170:1007–1015.
19. El-Bitar MK, Boustany RM. Common causes of uncommon seizures. Pediatr Neurol. 2009. 41:83–87.
20. Ekstrand JJ, Herbener A, Rawlings J, Turney B, Ampofo K, Korgenski EK, Bonkowsky JL. Heightened neurologic complications in children with pandemic H1N1 influenza. Ann Neurol. 2010. 68:762–766.
21. Yung M, Slater A, Festa M, Williams G, Erickson S, Pettila V, Alexander J, Howe BD, Shekerdemian LS. Australia and New Zealand Intensive Care Influenza Investigators and the Paediatric Study Group and the Clinical Trials Group of the Australia New Zealand Intensive Care Society. Pandemic H1N1 in children requiring intensive care in Australia and New Zealand during winter 2009. Pediatrics. 2011. 127:e156–e163.
22. Bailhache M, Sarlangue J, Castella C, Richer O, Fleury H, Koeck JL. Influenza A(H1N1)v virus infection in infants less than 6 months of age in southwestern France. Arch Pediatr. 2011. 18:383–389.
23. Kang JH, Oh CE, Lee J, Lee SY, Cha SH, Kim DS, Kim HH, Lee JH, Kim JT, Ma SH, Hong YJ, Cheong HJ, Lee HJ. Safety and immunogenicity of a new trivalent inactivated split-virus influenza vaccine in healthy Korean children: a randomized, double-blinded, active-controlled, phase III study. J Korean Med Sci. 2011. 26:1421–1427.
24. Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwane MK, Bridges CB, Grijalva CG, Zhu Y, Bernstein DI, Herrera G, Erdman D, Hall CB, Seither R, Griffin MR. The underrecognized burden of influenza in young children. N Engl J Med. 2006. 355:31–40.
25. O'Brien MA, Uyeki TM, Shay DK, Thompson WW, Kleinman K, McAdam A, Yu XJ, Platt R, Lieu TA. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children. Pediatrics. 2004. 113:585–593.
26. Chun JK, Cha BH, Uh Y, Kim HY, Kim YK, Kwon WC, Kim HM. The association of lymphopenia with the clinical severity in the Korean children admitted to the hospital with pandemic (H1N1) 2009 infection. Infect Chemother. 2011. 43:36–41.
27. Choi WS, Kim WJ, Cheong HJ. The evaluation of policies on 2009 influenza pandemic in Korea. J Prev Med Public Health. 2010. 43:105–108.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download