Abstract
Calcium pyrophosphate dihydrate (CPPD) deposition disease, also known as pseudogout, is a disease that causes inflammatory arthropathy in peripheral joints, however, symptomatic involvement of the intervertebral disc is uncommon. Herein, we describe a 59-yr-old patient who presented with cauda equina syndrome. Magnetic resonance imaging of the patient showed an epidural mass-like lesion at the disc space of L4-L5, which was compressing the thecal sac. Biopsy of the intervertebral disc and epidural mass-like lesion was determined to be CPPD deposits. We reviewed previously reported cases of pseudogout involving the lumbar intervertebral disc and discuss the pathogenesis and treatment of the disease.
Calcium pyrophosphate dihydrate (CPPD) deposition disease is defined as the deposition of CPPD crystals in the articular or periarticular structures that leads to inflammation of the joints (1, 2). It is characterized radiographically by chondrocalcinosis, heavy punctuate and linear radiodensities seen in both hyaline and fibrocartilage (2). It is known as pseudogout because of the clinical similarity to gouty arthritis (1). Pseudogout may occur in association with the following conditions: hemochromatosis, gout, diabetes mellitus, hyperparathyroidism, hypomagnesemia, hypothyroidism, osteoarthritis, and rheumatoid arthritis (3-5). Although mutations in the ankylosis protein homolog human gene have been shown recently to cause familial CPPD deposition disease, the exact cause is not well-established (6).
The clinical manifestations of pseudogout, which usually occur in elderly patients, range from an incidental finding to a destructive arthropathy (7). Pseudogout is a common disease causing inflammatory arthropathy in peripheral joints, and involvement of the spine is rare (1). Pseudogout of the spine is usually an incidental finding of the aging spine, and symptomatic involvement is much rarer (5). Although rare, pseudogout may affect all areas of the spine, with the majority of occurrences involving the ligamentum flavum (LF) and facet joint (1). Thus, symptomatic pseudogout involving the lumbar intervertebral disc is much rarer. Herein, we report on a case of pseudogout involving the lumbar intervertebral disc that caused cauda equina syndrome (CES) in a healthy patient.
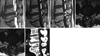
On September 19, 2011, a 59-yr-old male presented with severe lumbago and progressive neurological symptoms, including right-sided foot drop, numbness at both legs, and residual urine sensation. The patient felt a sudden onset of aggravated low back pain (LBP) after sleeping uncomfortably with leaning his body backward in his car seat 2 days prior to presentation. After that time, he experienced numbness in both legs, weakness on his right leg, urinary retention, and a worsening limping gait. He had undergone an uncomplicated L4-L5 discectomy 15 yr prior, and had a 2-month history of LBP, which was being managed conservatively. He had no arthralgia and no history of peripheral joint disease. His body temperature was 36.5℃ and he had no history of a recent febrile episode or febrile disease. On examination, he had weakness of 3/5 of the right ankle and big toe dorsiflexion, and saddle hypesthesia to light touch and pin-prick. A foley catheter was placed and 700 mL of residual urine was noted. Magnetic resonance imaging (MRI) of the lumbar spine revealed an extradural heterogeneous mass-like lesion in the anterior epidural space at the intervertebral disc space of L4-L5, which was severely compressing the thecal sac (Fig. 1). Computed tomography (CT) showed a patchy calcified lesion (Fig. 1). At this time, we did not know whether the lesion found on the MRI was a herniated disc with calcification or an extradural tumor.
Since we were under the impression that this was a case of CES, an emergent surgery was performed to decompress the thecal sac. We performed a total laminectomy at L4 and a posterior interbody fusion at L4-L5 was performed. During the operation, white to pink firm tissues in the anterior epidural space were found to be compressing the thecal sac. As we traced the margin of the lesion, we found some portion of the lesion was in continuity with the disk space through the annular defect. No neoplastic cells were found on the frozen pathological section. We assumed that the epidural mass-like lesion in the MRI was extruded, sequestered, and partially calcified nucleus pulposus. So after incision around the annular defect, we removed the epidural mass-like lesion and intervertebral disc all together in a piecemeal fashion. However, biopsy of the intervertebral disc and epidural mass-like lesion was determined to be CPPD deposits consistent with pseudogout (Fig. 2). Postoperatively, his symptoms of saddle hypesthesia and numbness on both legs were improved markedly. He regained his feeling of micturition and was able to void all urine on his own. He had no pain or abnormal calcifications on radiography of other joints. Results of blood tests, which included uric acid, calcium, phosphate, and a thyroid function test were all within normal limits. He was followed up for 6 months after the surgery. At the 6 month follow-up, his urination was nearly normal and his right ankle dorsiflexion had improved to a motor power of 4+/5.
Incidental or asymptomatic CPPD deposition in the lumbar intervertebral disc has been reported in the medical literature. In one review of lumbar spine radiographs, calcification was present in 21 of the 67 patients with idiopathic chondrocalcinosis articularis, but no association with back pain or stiffness was found (8). In addition, this finding has been reported at autopsy, as well as at surgery. From the results of the autopsy of 6 hemochromatosis patients with no pain or stiffness of the spine, CPPD deposition was found in 4 patients with degenerated disc (9). CPPD deposition was found in 3.1% to 12.6% of discs excised surgically in case of herniation or obtained from patients undergoing anterior lumbar interbody fusion (ALIF) (3, 10-12). Mohr et al. (11) suggested that CPPD deposition is an age-related phenomenon because there is an increased frequency of crystalline deposits with increasing age. Furthermore, in a study of Berleman et al. (12) with discs obtained during ALIF for severe discogenic pain, no differences were found in clinical features between patients with and without CPPD deposition.
Symptomatic cases have been rarely reported. In one review, there were 66 cases of symptomatic spinal pseudogout (1). Of these 66 cases, 40 were in the cervical spine, 6 in the thoracic spine, and 20 in the lumbar spine (1). Of the 20 lumbar spinal pseudogout cases, the majority involved the LF or facet joint, and 6 involved the intervertebral disc (1). In these 6 cases, the clinical presentation was intractable back pain and/or nerve root compression (5, 7, 13). In 2 of these 6 cases, in which MRI results were available, the lesions were a large extradural mass in the anterior epidural space that was severely compressing the root or the thecal sac that mimicked lumbar disc herniation or an extradural tumor (5, 7). The present case has similar MRI features to an extradural mass of the anterior epidural space compressing thecal sac, but is unique in that the patient presented with CES.
As previously indicated, CPPD deposition in the lumbar intervertebral disc is usually asymptomatic, and can occur in the absence of the clinical or radiographical features in other joints or associated systemic diseases, as in our patient (3, 5, 12). The clinical manifestations associated with the lumbar spine can occur without radiographical abnormalities on simple X-rays, as in our patient (5, 13). The role of surgery or trauma to the intervertebral disc has been suggested in CPPD deposition in the lumbar disc (3, 12-14). In Andres and Trainer's study (3), the significant common factor in all 7 patients with CPPD deposition in the intervertebral disc was a previous disc surgery at the same level, except one at adjacent level. In contrast, only 8% of those patients without CPPD deposition had undergone previous disc surgery in the same area (3). The increased incidence of prior surgery in the patients with CPPD deposition compared with the control group was statistically significant independent of sex and age (3). In a different study, 10 of 11 patients with intradiscal CPPD had a history of previous surgical intervention, and 9 of 11 patients recalled a specific incident or trauma that had initiated their back pain history; whereas, this was the case for only 54% of patients lacking CPPD deposition (12). In these studies, it was proposed that the surgical trauma might have played a role in the induction of CPPD deposition, as in our patient. Although it has been postulated that mechanical trauma to the joint cartilage or the ligaments is the initial event that leads to formation of these crystals and abnormal cartilage is the source of the pyrophosphate, whether trauma may be the inducement to crystal deposition remains unclear (13, 14). As the associated ingrowth of vascular or neurogenic tissue is lacking in the study of Berlemann et al. (12), a specific role for CPPD crystals in causing pain remains unproven. In our opinion, CPPD crystals per se are neither toxic to nor induce inflammatory reaction to nervous tissues. But in cases of mass formation adjacent to the nerve root or thecal sac or in case of herniation, it can compress the nerve root or thecal sac, and cause pain, radiculopathy, or CES. The time course of CPPD deposition in the lumbar intervertebral disc after trauma or surgery is unclear. In one study, the history of LBP ranged from 10 months to 27 yr after trauma or surgery (12). In our opinion, intradiscal CPPD deposition might be initiated and have progressed for a long time since a previous discectomy was performed 15 yr ago in the present case. However, herniation of CPPD deposited disc material into the epidural space caused compression of the thecal sac and the resultant CES.
Making a correct diagnosis is difficult because symptoms and signs resemble those of degenerative lumbar spinal disease and radiological features of chondrocalcinosis may not be present (1, 5). In pseudogout of the LF, CT may demonstrate the lesions as nodular or ovoid calcified lesions continuous with the lamina and MRI may demonstrate round or oval hypointense masses in the LF in both T1- and T2-weighted images (15, 16). However, no reports on the characteristics of pseudogout in the intervertebral disc in CT or MRI were found.
There are no pharmacological options to prevent CPPD deposition in tissues or to dissolve deposited crystals because much of the pathogenesis of CPPD deposition remains unclear. (15, 17). Currently, no evidence-based treatment guidelines are available, so treatment of CPPD deposition disease is restricted to symptomatic control (17). In this regard, surgical decompression should be requested in symptomatic cases resulting from the nerve root or thecal sac compression, as in the present case.
Although rare, pseudogout should be considered in the differential diagnosis of LBP, radiculopathy or CES. A high index of suspicion can help clinicians make the correct diagnosis, especially in patients with a history of prior disc surgery (1).
Figures and Tables
Fig. 1
Magnetic resonance imaging and computed tomography of the lumbar spine. (A) A T2-weighted sagittal image shows an epidural mass with heterogeneous signal intensity and the thecal sac is severely compressed by the mass. Focal low density (black arrows) in the lesion is suggestive of calcification. (B) Non-contrast and (C) contrast T1-sagittal image show enhancement (white arrows) in adjacent to the posterior longitudinal ligament. (D) Non-contrast T2- and (E) T1-weighted axial image show the thecal sac is severely compressed by the mass. (F) Computed tomography shows sparse calcification.
References
1. Lam HY, Cheung KY, Law SW, Fung KY. Crystal arthropathy of the lumbar spine: a report of 4 cases. J Orthop Surg (Hong Kong). 2007. 15:94–101.
2. Jeon CH, Choe WH, Ahn JK, Koh JH, Cha HS, Ahn JM, Koh EM. Calcium Pyrophosphate Dihydrate (CPPD) Crystal Deposition Disease Mimicking Meningitis: a case report and review of the literature. J Korean Rheum Assoc. 2001. 8:134–139.
3. Andres TL, Trainer TD. Intervertebral chondrocalcinosis: a coincidental finding possibly related to previous surgery. Arch Pathol Lab Med. 1980. 104:269–271.
4. Delamarter RB, Sherman JE, Carr J. Lumbar spinal stenosis secondary to calcium pyrophosphate crystal deposition (pseudogout). Clin Orthop Relat Res. 1993. 289:127–130.
5. Salcman M, Khan A, Symonds DA. Calcium pyrophosphate arthropathy of the spine: case report and review of the literature. Neurosurgery. 1994. 34:915–918.
6. Abhishek A, Doherty M. Pathophysiology of articular chondrocalcinosis--role of ANKH. Nat Rev Rheumatol. 2011. 7:96–104.
7. Baty V, Prost B, Jouvet A, Laurent J, Vallée B. Acute spinal cord compression and calcium pyrophosphate deposition disease. Case illustration. J Neurosurg. 2003. 99:2 Suppl. 240.
8. Richards AJ, Hamilton EB. Spinal changes in idiopathic chondrocalcinosis articularis. Rheumatol Rehabil. 1976. 15:138–142.
9. Bywaters EG, Hamilton EB, Williams R. The spine in idiopathic haemochromatosis. Ann Rheum Dis. 1971. 30:453–465.
10. Lagier R, Wildi E. Incidence of chondrocalcinosis in a series of 1,000 surgically excised intervertebral disks. Rev Rhum Mal Osteoartic. 1979. 46:303–307.
11. Mohr W, Oehler K, Hersener J, Wilke W. Chondrocalcinosis of the intervertebral disks. Z Rheumatol. 1979. 38:11–26.
12. Berlemann U, Gries NC, Moore RJ, Fraser RD, Vernon-Roberts B. Calcium pyrophosphate dihydrate deposition in degenerate lumbar discs. Eur Spine J. 1998. 7:45–49.
13. Ellman MH, Vazques LT, Brown NL, Mandel N. Calcium pyrophosphate dihydrate deposition in lumbar disc fibrocartilage. J Rheumatol. 1981. 8:955–958.
14. Doita M, Shimomura T, Maeno K, Nishida K, Fujioka H, Kurosaka M. Calcium pyrophosphate dihydrate deposition in the transverse ligament of the atlas: an unusual cause of cervical myelopathy. Skeletal Radiol. 2007. 36:699–702.
15. Muthukumar N, Karuppaswamy U. Tumoral calcium pyrophosphate dihydrate deposition disease of the ligamentum flavum. Neurosurgery. 2003. 53:103–108.
16. Brown TR, Quinn SF, D'Agostino AN. Deposition of calcium pyrophosphate dihydrate crystals in the ligamentum flavum: evaluation with MR imaging and CT. Radiology. 1991. 178:871–873.
17. Zhang W, Doherty M, Pascual E, Barskova V, Guerne PA, Jansen TL, Leeb BF, Perez-Ruiz F, Pimentao J, Punzi L, et al. EULAR recommendations for calcium pyrophosphate deposition. Part II: management. Ann Rheum Dis. 2011. 70:571–575.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download