Abstract
Risk factors for rickets of prematurity have not been re-examined since introduction of high mineral formula, particularly in ELBW infants. We analyzed the incidence and the risk factors of rickets in extremely low birth weight (ELBW) infants. As a retrospective case-control study from 2004 to 2008, risk factors were analyzed in 24 patients with rickets versus 31 patients without. The frequency of rickets in ELBW infants was 24/55 (44%). Infants with rickets were diagnosed at 48.2 ± 16.1 days of age, and improved by 85.3 ± 25.3 days. By radiologic evaluation, 29% were grade 1 rickets, 58% grade 2 and 13% grade 3. In univariate analysis, infants with rickets had significantly higher incidence of patent ductus arteriosus, parenteral nutrition associated cholestasis (PNAC), severe PNAC and moderate/severe bronchopulmonary dysplasia (BPD). In multiple regression analysis, after adjustment for gestation and birth weight, rickets significantly correlated with severe PNAC and with moderate/severe BPD. Serum peak alkaline phosphatase levels were significantly elevated in rickets (P < 0.001). In ELBW infants, the incidence of rickets of prematurity remains high and the incidence of severe PNAC and moderate/severe BPD was significantly increased 18 and 3 times, respectively.
In the 1980s, the prevalence of rickets in extremely low birth weight (ELBW) infants was around 50% and was 17% for fractures (1, 2). After introduction of a high mineral containing diet in the late 1980s, the prevalence decreased to 30% or lower (3, 4). There is little information, however, beyond 1985, concerning the incidence of rickets in extreme prematurity and factors that relate to its occurrence.
The pathogenesis of rickets in preterm infants is considered multifactorial. In addition to inadequate mineral intake as a major contributing factor (5), chronic co-morbidities, like chronic lung disease, short bowel syndrome, and parenteral nutrition associated cholestasis (PNAC) could also be related factors (6). Medications used in the neonatal intensive care unit (NICU), such as steroids, methylxanthines, and diuretics could theoretically increase the risk of inadequate bone mineralization (7-9). Other risk factors, such as immobilization from sepsis, cerebral pathology, muscular disorders and paralysis are associated with bone disease of prematurity (10). Since mineral enriched formula for the preterms began 30 yr ago, it is the standard practice to provide minerals in an attempt to reach in utero mineral accretion rates. In theory, under ideal conditions, the adventage of high mineral intake formulas should be associated with a marked reduction of rickets. However, it is often practically difficult to achieve ideal nutrition support in sick ELBW infants, the current incidence of rickets in ELBW infants is unknown, and the relationship of the severity of overall illness and degree of prematurity in this population is unclear.
The aim of this study was to characterize the risk factors for development of rickets in ELBW infants fed with high mineral containing preterm formula.
As a retrospective case-control study, medical records of 55 ELBW infants admitted into the Severance Children's Hospital NICU from 2004 to 2008 that survived until 1 month of age were reviewed. All infants had their serum alkaline phosphatase (ALP), calcium (Ca) and phosphate (P) levels reported weekly and bone screen radiography at 1 month of age. For nutritional care, fortified human milk (Ca 131 mg/100 kcal, P 71 mg/100 kcal) or a mineral fortified preterm formula (Ca 112-141 mg/kcal, P 52-63 mg/kcal) was used. During parenteral nutrition, Ca, P and multivitamins with trace minerals were added to parenteral nutrition, appropriately.
Rickets of prematurity was defined by radiologic screening criteria of Koo et al. (12). Grade 1 was defined as a loss of dense white line, increased lucency of bone shaft, and thinning of cortex; Grade 2 showed irregularity and fraying of a metaphysis with splaying and cupping; and Grade 3 presented evidence of fractures.
As co-morbidities, hyaline membrane disease (HMD), patent ductus arteriosus (PDA), necrotizing enterocolitis (NEC; using the modified Bell's staging criteria), parenteral nutrition associated cholestasis (PNAC, defined as direct bilirubin [DB] greater than 2.0 mg/dL), bronchopulmonary dysplasia (BPD; using NIH classification), retinopathy of prematurity (ROP, included above stage 2), and intraventricular hemorrhage (IVH, above grade 3) were analyzed. Severe PNAC was defined as elevation of DB greater than 4.0 mg/dL for more than 1 month and aspartate aminotransferase (AST) and alanine aminotransterase (ALT) greater than 60 IU/L and 35 IU/L, respectively. Moderate/severe BPD was defined in infants requiring either 28 days of supplemental oxygen therapy with oxygen, nasal continuous positive airway pressure (CPAP) or positive pressure ventilation at 36 weeks postmenstrual age or discharge, whichever came first, whose gestational age was younger than 32 weeks, or at 56 days postnatal age or discharge, whichever came first, in infants whose gestational age was older than 32 weeks. Statistics were calculated with the Student t-test and chi-square test for means and frequencies. Numeric data are presented as mean and standard deviation because the data were normally distributed. Calculations were performed with SPSS software version 17.0 (SPSS, Chicago, IL, USA). P values less than 0.05 were considered statistically significant.
Twenty-four ELBW infants (26.3 ± 1.9 weeks, 792 ± 99 g) were diagnosed as rickets of prematurity, and 31 infants without rickets (27.2 ± 2.3 weeks, 871 ± 93 g) were the control group. There was no significant difference of gestation and birth weight between the two groups. Rickets of prematurity was diagnosed in 43.9% of ELBW infants and fracture in 13%. For the rickets group, 7 infants (29%) showed G1, 14 infants (58%) G2 and 3 infants (13%) G3. Rickets of prematurity was diagnosed at 48.2 ± 16.1 days of life and improved by 85.3 ± 25.3 days of life.
In patient characteristics such as mean gestational ages, birth weight, gender, and frequency of intrauterine growth retardation (IUGR), there was no difference between the rickets and control groups. The cumulative amounts (either enteral or parenteral) of Ca and P administration during hospitalization were not different between the groups. For the rickets group in comparison with the control group, mean days on oxygen and ventilator use were significantly longer (P < 0.05). The duration of hospitalization and parenteral nutrition were significantly longer in the rickets group versus the control group (Table 1).
In univariate analysis, ELBW infants with rickets had a significantly higher occurrence of PDA (P = 0.025), PNAC (P = 0.041), severe PNAC (P = 0.013), BPD (P = 0.019), and moderate/severe BPD (P = 0.012) (Table 2). Common neonatal illnesses such as HMD, air leak, NEC, ROP, IVH and periventricular leucomalacia (PVL) were not different between the groups.
In multiple regression analysis after adjustment of gestation and birth weight, rickets in ELBW infants significantly correlated with severe PNAC (OR 18.5; 95% CI, 1.1-285; P = 0.042) and moderate/severe BPD (OR 3.2; 95% CI, 1.2-26.5; P = 0.04) (Table 3).
The mean level of peak ALP was significantly higher in the rickets group than the control group (952.2 ± 413.8 vs 524.7 ± 158.2 IU/L), while the mean level of the lowest Ca and P levels were not different between groups (Table 4).
In the 1980s, fortified mineral formulas were introduced to reduce the rates of rickets and fractures in very low birth weight (VLBW < 1,500 g) infants (11). Reports at that time indicated that up to 30% of VLBWs had rickets with fractures (12).
With advances in preterm nutrition, especially with the introduction of mineral fortified formulas, theoretically the incidence of rickets of prematurity should have dropped (3, 4). However, for increasing the survival of ELBW infants, significant co-morbid conditions could affect actual mineral intake which might mitigate the effect of high mineral fortification (13).
This is the first study to focus on the incidence of rickets in ELBW infants after 2 decades of fortified formula use in Korea. It is also the first to characterize and compare risk factors and neonatal morbidities for rickets of prematurity in ELBW infants. We found that the incidence of radiologic rickets in ELBW infants was extremely high at 44% (24/55) due to increased survival, and the incidence of significantly increased 18 times and 3 times for severe PNAC and moderate to severe BPD, respectively. Early recognition and treatment of active rickets may prevent subsequent bone damage and fractures.
Factors commonly thought to relate to rickets of prematurity include mismatch of postnatal intake of minerals compared to intrauterine mineral transfer, low bone loading effects in the NICU, intrauterine growth restriction, chorioamnionitis, steroids, methylxanthines and diuretic usage and necrotizing enterocolitis (7-10, 14-16). Inadequate mineral nutrient (calcium and phosphorous) intake appears to play a major role in causing rickets of prematurity (10, 17). Prolonged parenteral nutrition (PN) and delayed enteral nutrition complicate delivery of adequate mineral intake and would be particularly acute in ELBW infants (10). It is standard practice in NICU to provide high mineral preterm formula and human milk fortifier as an attempt to reach in utero mineral accretion rate, including the use of minimal early enteral feeding, which appears to improve bone mass at term (3, 17-19). However, in ELBW infants, co-morbid illnesses often preclude full enteral tolerance and the actual delivery of mineral nutrients (13). Further, when PN is needed, the provision of minerals in PN can be limited by solubility issues compounded by fluid restriction in sick infants (3).
Specific chronic co-morbidities, particularly chronic lung disease and short bowel syndrome which is generally associated with prolonged parenteral nutrition and PNAC, are potentially serious risk factors for poor bone mineralization (6, 20, 21). Thus our findings of markedly higher risk of rickets in ELBW infants with PNAC (18 times) and BPD (3 times) support these associations.
The limitations of this study are a small sample size of a single institution and a retrospective design. In conclusion, there is a high incidence (44%) of rickets in ELBW infants, which shows an 18-fold increase in severe PNAC and a 3-fold increase in moderate to severe BPD. We suggest that in ELBW infants with severe PNAC or moderate/severe BPD, aggressive prevention or treatment for rickets and fractures can be instituted earlier in the course of management.
Figures and Tables
Table 2
Comparison of co-morbidities between the rickets and control group
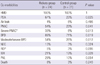
*DB ≧ 4.0 mg/dL for move than 1 month, and AST ≧ 60 IU/L, ALT 35 IU/L.HMD, hyaline membrane disease; PDA, patent ductus arteriosus; PNAC, parenteral nutrition associated cholestasis; BPD, bronchopulmonary dysplasia; NEC, necrotizing enterocolitis; ROP, retinopathy of prematurity; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia.
References
1. Lyon AJ, McIntosh N, Wheeler K, Williams JE. Radiological rickets in extremely low birthweight infants. Pediatr Radiol. 1987. 17:56–58.
2. McIntosh N, Livesey A, Brooke OG. Plasma 25-hydroxyvitamin D and rickets in infants of extremely low birthweight. Arch Dis Child. 1982. 57:848–850.
3. Greer FR. Osteopenia of prematurity. Annu Rev Nutr. 1994. 14:169–185.
4. MacDonald MG, Seshia MM, Mullett MD. Avery's neonatology: pathophysiology & management of the newborn. 2005. 6th ed. Philadelphia: Lippincott Williams & Wilkins.
5. Weber G, Guarneri MP, Corbella E, Gallia P, Chiumello G. Osteopenia in premature children: an emerging problem. Minerva Pediatr. 1989. 41:347–352.
6. Ferrone M, Geraci M. A review of the relationship between parenteral nutrition and metabolic bone disease. Nutr Clin Pract. 2007. 22:329–339.
7. Venkataraman PS, Han BK, Tsang RC, Daugherty CC. Secondary hyperparathyroidism and bone disease in infants receiving long-term furosemide therapy. Am J Dis Child. 1983. 137:1157–1161.
8. Weiler HA, Wang Z, Atkinson SA. Dexamethasone treatment impairs calcium regulation and reduces bone mineralization in infant pigs. Am J Clin Nutr. 1995. 61:805–811.
9. Zanardo V, Dani C, Trevisanuto D, Meneghetti S, Guglielmi A, Zacchello G, Cantarutti F. Methylxanthines increase renal calcium excretion in preterm infants. Biol Neonate. 1995. 68:169–174.
10. Harrison CM, Johnson K, McKechnie E. Osteopenia of prematurity: a national survey and review of practice. Acta Paediatr. 2008. 97:407–413.
11. American Academy of Pediatrics Committee on Nutrition. American Academy of Pediatrics Committee on Nutrition: nutritional needs of low-birth-weight infants. Pediatrics. 1985. 75:976–986.
12. Koo WW, Sherman R, Succop P, Krug-Wispe S, Tsang RC, Steichen JJ, Crawford AH, Oestreich AE. Fractures and rickets in very low birth weight infants: conservative management and outcome. J Pediatr Orthop. 1989. 9:326–330.
13. Rusk C. Rickets screening in the preterm infant. Neonatal Netw. 1998. 17:55–57.
14. Cakir M, Mungan I, Karahan C, Can G, Okten A. Necrotizing enterocolitis increases the bone resorption in premature infants. Early Hum Dev. 2006. 82:405–409.
15. Holland PC, Wilkinson AR, Diez J, Lindsell DR. Prenatal deficiency of phosphate, phosphate supplementation, and rickets in very-low-birth-weight infants. Lancet. 1990. 335:697–701.
16. Ryan S, Congdon PJ, James J, Truscott J, Horsman A. Mineral accretion in the human fetus. Arch Dis Child. 1988. 63:799–808.
17. Horsman A, Ryan SW, Congdon PJ, Truscott JG, James JR. Osteopenia in extremely low birthweight infants. Arch Dis Child. 1989. 64:485–488.
18. Rigo J, Pieltain C, Salle B, Senterre J. Enteral calcium, phosphate and vitamin D requirements and bone mineralization in preterm infants. Acta Paediatr. 2007. 96:969–974.
19. Steichen JJ, Gratton TL, Tsang RC. Osteopenia of prematurity: the cause and possible treatment. J Pediatr. 1980. 96:528–534.
20. Miller ME. The bone disease of preterm birth: a biomechanical perspective. Pediatr Res. 2003. 53:10–15.
21. Vestergaard P. Bone loss associated with gastrointestinal disease: prevalence and pathogenesis. Eur J Gastroenterol Hepatol. 2003. 15:851–856.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download