Abstract
The aim of this study was to describe in more detail the predisposition, natural course, and clinical impact of post-transplantation diabetes mellitus (PTDM) after heart transplantation (HT). The characteristics and clinical outcomes of 54 patients with PTDM were compared with those of 140 patients without PTDM. The mean age of PTDM patients was significantly higher than controls (48.9 ± 9.3 vs 38.6 ± 13.3 yr, respectively, P = 0.001), and ischemic heart disease was a more common indication of HT (20.4% [11/54] vs 7.1% [10/140], respectively, P = 0.008). In multivariate analysis, only recipient age (odds ratio, 1.80; 95% confidence interval, 1.35-2.40; P = 0.001) was associated with PTDM development. In 18 patients (33%), PTDM was reversed during the follow-up period, and the reversal of PTDM was critically dependent on the time taken to develop PTDM (1.9 ± 1.0 months in the reversed group vs 14.5 ± 25.3 months in the maintained group, P = 0.005). The 5-yr incidence of late infection (after 6 months) was higher in the PTDM group than in the control group (30.4% ± 7.1% vs 15.4% ± 3.3%, respectively, P = 0.031). However, the 5-yr overall survival rate was not different (92.9% ± 4.1% vs 85.8% ± 3.2%, respectively, P = 0.220). In conclusion, PTDM after HT is reversible in one-third of patients and is not a critical factor in patient survival after HT.
From the early days of transplantation, post-transplantation diabetes mellitus (PTDM) has been recognized as a major complications of solid organ transplantation. Although its incidence has decreased with efforts to lower the dose of steroid used, PTDM continues to be prevalent in solid organ transplantation (1, 2). As with pre-existing diabetes, PTDM was thought to have a potential impact in post-transplantation outcomes. PTDM has been shown to be an independent risk factor of graft failure, cardiovascular disease, and death in kidney (3-5) and liver transplantation (6, 7). However, only a small number of studies have investigated the predisposing factors of PTDM in heart transplantation (HT) (8-11). Regarding its impact on the natural course and prognosis of HT, only two previous studies were published and showed no differences in the incidence of coronary vasculopathy and mortality after HT (10, 12). However, these studies were limited by small patient numbers and were brief analyses incidental to the main research. Understanding of natural course and impact of PTDM is important for its proper screening and treatment. Indeed, since tacrolimus (TAC) has been used widely and shown to be more diabetogenic than cyclosporine (CSA) in kidney transplantation (4, 13), the clinical importance of PTDM has emerged. In this study, we sought to describe in more detail the predisposition, natural course, and clinical impact of PTDM after HT.
From May 1997 to May 2010, 222 patients underwent HT in our institute. The main study cohorts consisted of 54 patients with PTDM (PTDM group) and 140 patients without PTDM (control group). All patients were followed at the same institute after HT. Those patients were followed up monthly for 12 months, and every three months thereafter. Complicated patients were followed more frequently based on the attending physician's discretion. Follow-up data were collected by direct contact, telephone interview, or detailed medical record review. The mean follow-up duration was 64.3 ± 43.4 months for the PTDM group and 75.3 ± 50.2 months for the control group. Follow-up data of 28 patients with pre-existing diabetes mellitus (DM) was also collected for separate analysis (DM group, mean follow-up period 55.1 ± 48.5 months).
PTDM was diagnosed using the American Diabetes Association criteria (14). In our institute, fasting blood glucose (FBG) levels were tested during every outpatient visit after HT for surveillance and follow-up of PTDM. Patients were diagnosed with PTDM when the FBG level was 126 mg/dL or greater and confirmed by repeated tests during outpatient follow-up visits. Oral glucose tolerance tests were conducted in patients suspicious for diabetes despite a FBG level below 126 mg/dL. Episodes of induced transient hyperglycemia due to high-dose steroid use or steroid pulse therapy for rejection, immediate post operative period, intravenous dextrose or nutritional therapy and significant infectious disease were excluded from PTDM diagnosis. PTDM patients were prescribed insulin and/or oral hypoglycemic agents with a target HbA1C < 7.0 and target fasting glucose < 130 mg/dL (15). The term "reversed PTDM" was assigned to patients who no longer needed insulin and/or oral hypoglycemic agents and who had adequate glucose and HbA1C control. The time to PTDM development was defined as the time from HT to diagnosis at the outpatient clinic.
Interleukin-2 monoclonal antibody (Basliximab or Daclizumab) induction therapy has been used since 2000. Maintenance of an immunosuppressive protocol consisted of a triple combination of a steroid, calcineurin inhibitor (CSA or TAC), and anti-proliferative agent (azathioprine or mycophenolate mofetil). The steroid was given intravenously at an initial dose of 1 mg/kg methylprednisolone, tapered to 10 mg/day by the time of discharge, and adjusted to a maintenance dose of 5 mg/day within 6 months after HT. Withdrawal or continuance of the steroid (at the maintenance dose) was based on the attending physician's discretion and the patient's clinical status. The calcineurin inhibitor was routinely prescribed to all patients. CSA was the major calcineurin inhibitor used until 2007, and TAC was predominantly used after 2007. The calcineurin inhibitor dose on the second day of HT and the initial target trough concentration were 200-350 ng/mL for CSA and 10-15 ng/mL for TAC. The target trough concentration was adjusted between 100 and 200 ng/mL for CSA and between 5 and 10 ng/mL for TAC 6 months after HT. Azathioprine (AZA) was the major anti-proliferative agent used until 1999, and mycophenolate mofetil replaced AZA after 1999.
We evaluated CAV using the standardized protocol of our institute. All patients underwent baseline coronary angiography (CAG) with intravascular ultrasound 1 month after HT. Baseline CAG data were available in 91.3% (177 of 194) of patients. Surveillance CAG was performed first 1 yr after HT and thereafter every other year. CAG was also performed whenever the clinical situation suggested CAV. We categorized the degree of CAV according to ISHLT criteria (16). The study outcome "occurrence of CAV" was defined as any angiographic abnormality. Newly developed CAG and progression from baseline CAG were noted on surveillance CAG.
All patients underwent serial endomyocardial biopsies at regular intervals. The ISHLT grading system was used to define the degree of rejection and to make a decision regarding use of immunosuppressive therapy (17). A transient increase in the corticosteroid dose with or without steroid pulse therapy was prescribed in the event of acute rejection grade 2R or 3R. The study outcome "episode of rejection" was defined as pathologically confirmed rejection of ISHLT grade 2R or greater needing steroid dose-up or steroid pulse therapy.
Serologic surveillance for Cytomegalovirus, Herpes simplex virus, Varicella-zoster virus, and Epstein-Bar virus was performed before HT. After HT, surveillance via blood culture, urine, and sputum analysis was performed weekly for 1 month. The Cytomegalovirus antigenemia assay was performed weekly during hospitalization and during every visit at the outpatient clinic until 1 yr after HT. Gancyclovir prophylaxis was prescribed for all patients. Trimethoprime/sulfomethoxazole prophylaxis was prescribed for all patients for 1 yr after HT. The study outcome "episode of infectious disease" was defined as any infectious disease needing hospitalization and therapeutic intervention. We divided episodes of infectious disease into three periods: immediate (before 1 month), early (1-6 months), and late (after 6 months) infection after HT.
All statistical analyses were performed with SPSS (version 18.0; SPSS, Inc., Chicago, IL, USA). Continuous variables are presented as mean ± SD values. Statistical analysis of the differences between the groups was completed using unpaired Student's t test. The chi-square test and Fisher's exact test were used to compare the frequency of categorical variables. Multivariable logistic regression with backward elimination was used to determine predisposing factors of PTDM. Kaplan-Meier survival analysis was used to compare survival function and clinical event incidence. All P values were two-sided, and a value of P < 0.05 was considered significant.
Patient characteristics are summarized in Table 1. Patients with PTDM were significantly older than control patients (48.9 ± 9.3 vs 38.6 ± 13.3 yr, respectively, P = 0.001) and had a higher prevalence of ischemic cardiomyopathy (ICMP) as an indication for transplantation (20.4% [11/54] vs 7.1% [10/140], respectively, P = 0.008). Donor age in the PTDM group was greater than in the control group (32.4 ± 8.9 vs 29.4 ± 9.8 yr, respectively, P = 0.048). Body mass index (BMI), renal function, and lipid profile before HT did not differ significantly between the groups. During HT, ischemic time of heart was significantly longer in the PTDM group than in the control group (163.3 ± 61.0 vs 140.4 ± 55.6 min, respectively, P = 0.015). After transplantation, there was a trend of more TAC-based immunosuppressant use in the PTDM group than in the control group (38.9% [21/54] vs 25.7% [36/140], respectively, P = 0.080), although statistical significance was not reached. The steroid tapering schedule and rate of steroid discontinuation were not different between the groups.
To determine the predictors of PTDM, we performed multivariate analysis using a logistic regression model (Table 2). In univariate analysis, recipient age (odds ratio [OR], 1.83; 95% confidence interval [CI], 1.38-2.43, P = 0.001), donor age (OR, 1.39; CI, 1.01-1.89, P = 0.039), ICMP as an indication of HT (OR, 3.33; CI, 1.32-8.37, P = 0.011), and ischemic time during HT (OR, 1.07; CI, 1.01-1.12, P = 0.019) were significantly associated with PTDM occurrence. Recipient sex, donor age, and TAC-based immunosuppression were marginally associated with PTDM, but not with statistical significance. In multivariate analysis, only recipient age (OR, 1.80; 95% CI, 1.35-2.40, P = 0.001) was significantly associated with PTDM occurrence.
Most PTDM cases (72.2% [39/54]) were diagnosed during the first outpatient visit after HT (Fig. 1). PTDM patients were already prescribed insulin or an oral hypoglycemic agent before this visit. The mean time to PTDM development was 10.3 ± 21.5 months. During follow-up, 61.1% (33/54) of PTDM patients were treated by oral hypoglycemic agent and 38.9% (21/54) required insulin. The mean FBG level at the first outpatient visit was 143.9 ± 36.1 mg/dL. After treatment, mean FBG level was decreased to 113.9 ± 22.8 mg/dL at one year after HT and 112.9 ± 28.6 mg/dL at their last follow-up. The mean HbA1C level at initial outpatient visit was 8.1% ± 1.8% and decreased to 6.9% ± 0.9% at one year and 7.0% ± 0.9% at their last follow-up.
PTDM was reversed in 18 (33%) patients during the follow-up period, and the mean time to PTDM reversal was 21.3 ± 22.5 months. Patient characteristics of reversed and non-reversed PTDM are summarized in Table 3. Reversed PTDM occurred significantly earlier than non-reversed PTDM (1.9 ± 1.0 vs 14.5 ± 25.3 months, respectively, P = 0.005), whereas other factors were not statistically significant. Fig. 1 shows the temporal pattern of PTDM occurrence. PTDM reversal was critically dependent on the time to PTDM development. PTDM was not reversed in patients in whom PTDM occurred more than 6 months after HT.
The 5-yr overall survival rate was not significantly different between PTDM and control patients (Fig. 2A; 92.9% ± 4.1% vs 85.8% ± 3.2%, respectively, P = 0.220). Non-cardiac death was more prevalent than cardiac death in both groups, and the cause of death was not significantly different between the groups (Table 4). The 5-yr CAV incidence rate (Fig. 2B; 21.9% ± 8.7% vs 19.0% ± 4.8%, respectively, P = 0.420) and rejection episodes (Fig. 2C; 13.0% ± 4.6% vs 19.6% ± 3.4%, respectively, P = 0.302) were not different between the PTDM and the control groups. During the immediate postoperative period, infection episodes were more prevalent in the PTDM group than in the control group (Fig. 2D; 11.1% [6/54] vs 2.9% [4/140], respectively, P = 0.030). The 5-yr incidence of late infection (after 6 months) was higher in the PTDM group than in the control group (30.4% ± 7.1% vs 15.4% ± 3.3%, respectively, P = 0.031). There were more episodes of bacterial (25.9% [14/54] vs 10.7% [15/140], respectively, P = 0.008) and fungal (13% [7/54] vs 1.4% [2/140], respectively, P = 0.002) infections in the PTDM group than in the control group. Episodes of viral and mycobacterial infections were not significantly different between the groups (Table 5). In terms of the infection site, pneumonia was more prevalent in the PTDM group than in the control group (27.8% [15/54] vs 10.7 [15/140], respectively, P = 0.003), whereas no differences were found in other sites (Table 6).
To determine the clinical impact of reversal of PTDM, further analysis for study outcomes was performed between reversed (n = 18) and non-reversed (n = 36) PTDM group. During 5 yr, there were no significant difference in terms of overall survival rate (94.4% ± 5.4% vs 91.1% ± 6.4%, respectively, P = 0.896), incidence of CAV (16.9% ± 10.9% vs 29.2% ± 14.8%, respectively, P = 0.775), incidence of rejection (5.6& ± 5.4% vs 16.7% ± 6.2%, respectively, P = 0.255), and incidence of infectious episodes during the immediate (16.7% [3/18] vs 8.3% [3/36], respectively, P = 0.358) and early (16.7% [3/18] vs 19.4% [7/36], respectively, P = 0.804) post-operative period. The incidence of late infection (13.7% ± 9.2% vs 38.0% ± 9.1%, respectively, P = 0.069) was slightly higher in non-reversed group than reversed group, although statistical significance was not reached.
Among the patients with pre-existing DM, mean time interval between diagnosis of DM and HT was 8.0 ± 6.6 yr. Compared to the subjects of the PTDM group, those patients in pre-existing DM group were older (48.9 ± 9.3 vs 56.0 ± 6.1 yr old, respectively, P = 0.001) and lesser male patients were included in DM group (81.5% [44/54] vs 64.3% [18/28], respectively, P = 0.058). Frequency of ICMP (20.4% [11/54] vs 14.3% [4/28]), TAC based regimen (38.9% [21/54] vs 35.7% [10/28]), and steroid withdrawal (38.9% [21/54] vs 32.1% [9/28]) were not significantly different. Also, baseline BMI (22.7 ± 3.5 vs 22.5 ± 2.9), total cholesterol level (135.8 ± 41.9 vs 145.8 ± 31.2 mg/dL), triglyceride level (79.9 ± 41.8 vs 99.7 ± 53.9 mg/dL), and serum creatinine level (1.3 ± 0.6 vs 1.1 ± 0.4 mg/dL) were not significantly different. During 5 yr, there was no significant difference between PTDM and DM group in terms of overall survival (92.9% ± 4.1% vs 88.6% ± 6.2%, respectively, P = 0.141), incidence of CAV (21.9% ± 8.7% vs 35.7% ± 21.0, respectively, P = 0.924), and incidence of rejection (12.9% ± 4.6% vs 17.9% ± 7.2%, respectively, P = 0.308). Also, incidence of infectious episode during immediate (11.1% [6/54] vs 17.9% [5/28], P = 0.299), early (18.5% [10/54] vs 10.7% [3/28], respectively, P = 0.359), and late (30.4% ± 7.1% vs 29.1% ± 11.6%, respectively, P = 0.818) period were not significantly different.
In this study of the HT registry of one Korean hospital, we found several important findings about PTDM after HT. The most important predisposing factor for PTDM development was recipient age. Most PTDM occurred during the early post-operative period, especially during the first 6 months. During follow-up, PTDM was reversed in one-third of patients, and its reversal was critically dependent on the time to PTDM development. PTDM had no significant impact on overall survival, incidence of CAV, and acute rejection. However, PTDM patients experienced more immediate post-operative and late infectious episodes.
The incidence of PTDM in our study was 27.8% during 6-yr follow-up, which was not very different than data of the largest ISHLT registry (-20% at 10 yr) (18). In previous studies focused on PTDM development, the incidence was 13.5%-39.7%, and the important predisposing factors for PTDM were age, BMI, post-operative steroid dose, glucose intolerance before HT, TAC treatment, and episodes of rejection (8-11). In our study, recipient age was the only significant predisposing factor for PTDM. It was not a surprising finding considering that age is one of the most important risk factors of DM (15). However, other risk factors of DM, especially the components of metabolic syndrome (BMI, triglyceride, and cholesterol) were not different between PTDM and control patients. The exact cause of this insignificance of metabolic components could not be understood using this registry data, but it could be partially explained by the difference in ethnicity. Although the exact mechanism has not been proven, the impairment of insulin secretion rather than insulin resistance is known to be the initial abnormality in the development of DM in Korean patients (19). Also, most Korean type 2 DM patients are not obese, and BMI and waist-to-hip ratio were not significant risk factors in Korean population-based studies (20, 21). Although this hypothesis is limited due to the lack of direct comparison data between Koreans and Caucasians, it has clinical implications for the management of oriental patients undergoing HT.
TAC use was not an independent predictor in our study. However, we cannot state that TAC had no effect on PTDM, because the follow-up period of the TAC-based regimen was too short to compare to CSA. Also, the dose and tapering schedule of the prescribed steroid were not different between the groups, because the same steroid tapering protocol was applied to all patients. One interesting finding is that the ischemic time was marginally associated with PTDM, although statistical significance was not reached. The prolonged ischemic time of a cardiac allograft is known to be related to poor early graft performance, longer ICU stay, and other organ dysfunction (22, 23). Although it was not statistically significant, the relationship between pancreatic beta cell dysfunction and ischemic time could be a clinically possible scenario and efforts to reduce ischemic time may be of benefit to patients.
One of the interesting findings of our study is that PTDM was reversed in one-third of patients. The reversal of PTDM was reported in previous liver or kidney transplantation studies (24-26), but there were limited data about the characteristics of reversed PTDM. In patients with reversed (versus non-reversed) PTDM, a trend for higher high density lipoprotein cholesterol level, lower ICMP, and lower TAC use was observed. However, the most distinguishing and only statistically significant characteristic was the time to PTDM development. Only early onset PTDM was reversed. This time course dependency may suggest a difference of pathogenesis between PTDM occurring before versus after 6 months. We thought the most important difference between PTDM occurring before versus after 6 months may be the dose of steroid used, which was tapered to or lower than the maintenance dose (5 mg of prednisolone) after 6 months. Therefore, we hypothesized that early PTDM occurring before 6 months was triggered by high dose steroid use and had some potential for reversal. Conversely, PTDM occurring after 6 months may be a de novo type 2 DM, because the diabetogenicity of a lower than maintenance dose was minimal in a previous study (27). This finding may indicate that a different approach is needed for managing early PTDM versus late PTDM. The possibility of reversal of early DM warrants continuous monitoring of glucose status and caution again overzealous hypoglycemic treatment.
PTDM presence had no influence on the probability of patient survival, as was similarly shown by Klingenberg et al. (12). The lack of a difference in the survival rate could be supported by the large study of Russo et al. (28), which enrolled more than 20,000 patients among whom were more than 3000 DM patients. In that study, patients with uncomplicated DM had a similar survival rate compared with patients without DM, whereas patients with complicated DM showed increased mortality as DM-related complications increased. Because PTDM is a newly diagnosed DM and usually treated intensively, patients with PTDM could have a similar natural course as those with well-controlled uncomplicated DM. Also, PTDM had no influence on vasculopathy or rejection in our patients. However, PTDM increased the patient infection rate. The association of pre-transplant DM and infection has already been well studied by Marelli et al., who showed higher 90-day and 4-yr infection rates in DM patients (29). The impaired immune response in the diabetic state (30) and the baseline characteristics (more elders and ICMP) of PTDM patients may affect the increasing infection rate. This increased infection risk did not translate to differences in survival. Nevertheless, because PTDM patients showed more susceptibility to pneumonia in our study, more intensive surveying for infectious disease presence may be needed.
Although we tried to include a large number of HT patients, it is difficult to generalize our findings because the study was a single center study of Asian patients. The numbers of PTDM and reversed PTDM patients were too small to determine the statistically significant predictors of reversal. Also, relatively small study population and short follow-up period could bring some selection bias and beta-errors. But, our study population is the largest HT registry in the Korea. Furthermore, follow-up duration of our study was longer than previous studies (10, 12) and outcome data were reliable because all the study participants were followed regularly in a single center without loss. Despite limitations described above, we believe that our data could represent the real-world clinical impact of PTDM in Korean HT patients. TAC was available to patients with HT only after 2007 in Korea. As a result, the follow-up period of TAC patients was significantly shorter than CSA patients, and it is difficult to assess its impact on PTDM occurrence and reversal. Although we enrolled patients undergoing HT before 2010, the incidence of PTDM could be underestimated because there were some patients who developed PTDM several years after HT. Also, the number of reversed PTDM patients could be increased if the follow-up period was extended.
In conclusion, PTDM is reversible in one-third of patients and not a critical factor of patient survival after HT. However, more intensive surveying for infectious diseases and optimal glucose control is warranted in these patients.
Figures and Tables
Fig. 2
The clinical impact of PTDM. (A) Five-year overall survival rate; (B) Five-year cardiac allograft vasculopathy incidence; (C) Five-year rejection incidence; (D) Immediate, early, and late infection incidence after HT.

Table 1
Baseline characteristics of patients
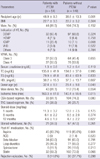
BMI, body mass index; HT, heart transplantation; DCMP, dilated cardiomyopathy; ICMP, ischemic cardiomyopathy; HCMP, hypertrophic cardiomyopathy; VHD, valvular heart disease; NYHA, New York Heart Association functional class; TC, total cholesterol; TG, triglyceride; HDL, high density lipoprotein cholesterol; TAC, tacrolimus; CSA, cyclosporine A; ACEi, angiotensin converting enzyme inhibitor.
References
1. Gunnarsson R, Arner P, Lundgren G, Magnusson G, Ostman J, Groth CG. Diabetes mellitus: a more-common-than-believed complication of renal transplantation. Transplant Proc. 1979. 11:1280–1281.
2. Bodziak KA, Hricik DE. New-onset diabetes mellitus after solid organ transplantation. Transpl Int. 2009. 22:519–530.
3. Hjelmesaeth J, Hartmann A, Leivestad T, Holdaas H, Sagedal S, Olstad M, Jenssen T. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. 2006. 69:588–595.
4. Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003. 3:178–185.
5. Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, Ferguson RM. Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int. 2002. 62:1440–1446.
6. Baid S, Cosimi AB, Farrell ML, Schoenfeld DA, Feng S, Chung RT, Tolkoff-Rubin N, Pascual M. Posttransplant diabetes mellitus in liver transplant recipients: risk factors, temporal relationship with hepatitis C virus allograft hepatitis, and impact on mortality. Transplantation. 2001. 72:1066–1072.
7. John PR, Thuluvath PJ. Outcome of patients with new-onset diabetes mellitus after liver transplantation compared with those without diabetes mellitus. Liver Transpl. 2002. 8:708–713.
8. Mogollón Jiménez MV, Sobrino Márquez JM, Arizón Muñoz JM, Sánchez Brotons JA, Guguisa Rasco A, Hernández Jiménez MM, Romero Rodríguez N, Borrego Domínguez JM, Ordoñez Fernández A, Lage Gallé E, et al. Incidence and importance of de novo diabetes mellitus after heart transplantation. Transplant Proc. 2008. 40:3053–3055.
9. Martínez-Dolz L, Almenar L, Martínez-Ortiz L, Arnau M, Chamorro C, Moro J, Osa A, Rueda J, Garcia C, Palencia M. Predictive factors for development of diabetes mellitus post-heart transplant. Transplant Proc. 2005. 37:4064–4066.
10. Depczynski B, Daly B, Campbell LV, Chisholm DJ, Keogh A. Predicting the occurrence of diabetes mellitus in recipients of heart transplants. Diabet Med. 2000. 17:15–19.
11. Nieuwenhuis MG, Kirkels JH. Predictability and other aspects of post-transplant diabetes mellitus in heart transplant recipients. J Heart Lung Transplant. 2001. 20:703–708.
12. Klingenberg R, Gleissner C, Koch A, Schnabel PA, Sack FU, Zimmermann R, Katus HA, Dengler TJ. Impact of pre-operative diabetes mellitus upon early and late survival after heart transplantation: a possible era effect. J Heart Lung Transplant. 2005. 24:1239–1246.
13. Vincenti F, Friman S, Scheuermann E, Rostaing L, Jenssen T, Campistol JM, Uchida K, Pescovitz MD, Marchetti P, Tuncer M, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007. 7:1506–1514.
14. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011. 34:S62–S69.
15. American Diabetes Association. Standards of medical care in diabetes: 2011. Diabetes Care. 2011. 34:S11–S61.
16. Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, Madsen J, Parameshwar J, Starling RC, Uber PA. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010. 29:717–727.
17. Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005. 24:1710–1720.
18. Taylor DO, Stehlik J, Edwards LB, Aurora P, Christie JD, Dobbels F, Kirk R, Kucheryavaya AY, Rahmel AO, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Heart Transplant Report-2009. J Heart Lung Transplant. 2009. 28:1007–1022.
19. Kim DJ, Lee MS, Kim KW, Lee MK. Insulin secretory dysfunction and insulin resistance in the pathogenesis of Korean type 2 diabetes mellitus. Metabolism. 2001. 50:590–593.
20. Lee TH. Prevalence of obesity in Korean non-insulin-dependent diabetic patients. Diabetes Res Clin Pract. 1996. 32:71–80.
21. Shin CS, Lee HK, Koh CS, Kim YI, Shin YS, Yoo KY, Paik HY, Park YS, Yang BG. Risk factors for the development of NIDDM in Yonchon County, Korea. Diabetes Care. 1997. 20:1842–1846.
22. Marasco SF, Esmore DS, Richardson M, Bailey M, Negri J, Rowland M, Kaye D, Bergin PJ. Prolonged cardiac allograft ischemic time: no impact on long-term survival but at what cost? Clin Transplant. 2007. 21:321–329.
23. Rylski B, Berchtold-Herz M, Olschewski M, Zeh W, Schlensak C, Siepe M, Beyersdorf F. Reducing the ischemic time of donor hearts will decrease morbidity and costs of cardiac transplantations. Interact Cardiovasc Thorac Surg. 2010. 10:945–947.
24. Hjelmesaeth J, Hartmann A, Kofstad J, Egeland T, Stenstrøm J, Fauchald P. Tapering off prednisolone and cyclosporin the first year after renal transplantation: the effect on glucose tolerance. Nephrol Dial Transplant. 2001. 16:829–835.
25. Romagnoli J, Citterio F, Violi P, Cadeddu F, Nanni G, Castagneto M. Post-transplant diabetes mellitus: a case-control analysis of the risk factors. Transpl Int. 2005. 18:309–312.
26. Navasa M, Bustamante J, Marroni C, González E, Andreu H, Esmatjes E, Garcia-Valdecasas JC, Grande L, Cirera I, Rimola A, et al. Diabetes mellitus after liver transplantation: prevalence and predictive factors. J Hepatol. 1996. 25:64–71.
27. Midtvedt K, Hjelmesaeth J, Hartmann A, Lund K, Paulsen D, Egeland T, Jenssen T. Insulin resistance after renal transplantation: the effect of steroid dose reduction and withdrawal. J Am Soc Nephrol. 2004. 15:3233–3239.
28. Russo MJ, Chen JM, Hong KN, Stewart AS, Ascheim DD, Argenziano M, Mancini DM, Oz MC, Naka Y. Survival after heart transplantation is not diminished among recipients with uncomplicated diabetes mellitus: an analysis of the United Network of Organ Sharing database. Circulation. 2006. 114:2280–2287.
29. Marelli D, Laks H, Patel B, Kermani R, Marmureanu A, Patel J, Kobashigawa J. Heart transplantation in patients with diabetes mellitus in the current era. J Heart Lung Transplant. 2003. 22:1091–1097.
30. Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999. 26:259–265.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download