Abstract
Few studies have included magnetoencephalography (MEG) when assessing the diagnostic value of presurgical modalities in a nonlesional epilepsy population. Here, we compare single photon emission computed tomography (SPECT), positron emission tomography (PET), video-EEG (VEEG), and MEG, with intracranial EEG (iEEG) to determine the value of individual modalities to surgical decisions. We analyzed 23 adult epilepsy patients with no abnormal MRI findings who had undergone surgical resection. Localization of individual presurgical tests was determined for hemispheric and lobar locations based on visual analysis. Each localization result was compared with the ictal onset zone (IOZ) defined by using iEEG. The highest to the lowest hemispheric concordance rates were MEG (83%) > ictal VEEG (78%) > PET (70%) > ictal SPECT (57%). The highest to lowest lobar concordance rates were ictal VEEG = MEG (65%) > PET (57%) > ictal SPECT (52%). Statistical analysis showed MEG to have a higher hemispheric concordance than that of ictal SPECT (P = 0.031). We analyzed the effects of MEG clustered-area resection on surgical outcome. Patients who had resection of MEG clusters showed a better surgical outcome than those without such resection (P = 0.038). It is suggested that MEG-based localization had the highest concordance with the iEEG-defined IOZ. Furthermore, MEG cluster resection has prognostic significance in predicting surgical outcome.
It is well known that the presence of a specific lesion predicts a favorable surgical outcome (1-3). However, about 20%-30% of refractory epilepsy patients show no lesions in magnetic resonance (MR) images (4, 5), and only about 40% of them achieve seizure freedom after surgery (6-8). The diagnostic values of various presurgical evaluation modalities have been studied separately in nonlesional epilepsy populations (6, 9-11). However, few studies have concurrently compared various modalities (12). Moreover, even though magnetoencephalography (MEG) is a useful tool for epileptogenic zone localization, few electrophysiological studies have investigated MEG for its diagnostic value in a nonlesional epilepsy population, especially in an adult group. In a study into the use of MEG in nonlesional epilepsy, Garcia-Morales et al. only included four children with startle epilepsy (13). In another MEG study which included 22 patients; all subjects were children in which the authors compared scalp electroencephalography (EEG) with intracranial EEG (iEEG), not with other modalities, such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT) (14). Seo et al. compared various presurgical modalities, including SPECT, PET, scalp EEG, and iEEG, within one study; however, their patients were also limited to children (12). Regarding studies that include an adult nonlesional epilepsy population, one study focused on reevaluation of MR imaging (MRI) results using MEG results (15), while another study only compared MEG results with those of invasive EEG (16). The purpose of the present study is, therefore, to compare findings from various presurgical evaluation modalities, including SPECT, PET, video EEG (VEEG), and MEG, with those from iEEG to determine the relative value of those individual modalities to surgical decisions on nonlesional neocortical epilepsy in adult patients.
Initially included were 37 adult patients with intractable epilepsy with no abnormal MRI findings who underwent MEG examination and resective surgery between 2005 and 2011 at Seoul National University Hospital, Korea. Of that total, 8 patients who had fewer than 3 MEG interictal spikes, 2 patients with less than a 1 yr follow-up period, and 4 patients who had hippocampal sclerosis or tumor, based on pathology examination results, were excluded. As a result, 23 patients (mean age at surgery = 30.7 yr; standard deviation [SD] = 9.0; 10 females) were included in our retrospective study of nonlesional neocortical epilepsy. The mean postoperative follow-up period was 2.9 yr (SD = 1.3).
All patients were examined using either a GE 1.5 T or 3 T MRI system (GE Horizon Echospeed; GE Healthcare, Little Chalfont, UK) or a Siemens 1.5 T scanner (MAGNETOM Avanto; Siemens, Erlangen, Germany). Our standard MRI protocol included T2 and fluid attenuated inversion recovery (FLAIR) axial, T2 and FLAIR oblique coronal, fast inversion recovery with myelin suppression (FIRMS), and three dimensional (3D) gradient echo coronal T1 images with whole brain coverage. The 3D gradient echo T1 images were reconstructed to a slice thickness of 1 mm, while the T2 images were acquired by using a 3 mm thickness with a 1 mm inter-slice gap. Preoperative MRI results were reviewed separately and confirmed by two neuroradiologists specializing in epilepsy and blinded to seizure focus.
Both interictal PET and ictal SPECT were performed in all patients (6). The FDG-PET and ictal SPECT scans were reviewed by a nuclear medicine specialist blinded to seizure focus.
Both scalp VEEG and invasive iEEG were done in all patients. Ictal scalp VEEG data were recorded by using a VEEG monitoring system (Grass-Telefactor, West Warwick, RI, USA) with electrodes placed according to the International 10-20 system and with additional anterior temporal electrodes. We used a combination of grids and strips for iEEG. Grid and strip placements were determined on the basis of results of presurgical evaluations including ictal scalp VEEG, PET, ictal SPECT, and clinical semiology. The signals were recorded with a bandpass filter of 0.3-70 Hz and digitized at 200 Hz. At least three habitual seizures were recorded during scalp VEEG and iEEG monitoring. Both scalp VEEG and iEEG results were reviewed by two epileptologists. The intracranial ictal onset zone (IOZ) was defined as the area with the first sustained rhythmic change in EEG results that could be differentiated from background and interictal waves.
Spontaneous cortical magnetic activity and EEG activities were simultaneously recorded over approximately 60 min using a whole-head MEG system (VectorView™; Elekta Neuromag) consisting of 306 channels arranged in triplets of two planar gradiometers and one magnetometer. The location of the subject's head in the MEG sensor space was determined by measuring the magnetic signals from four head-position indicator coils placed on the scalp. The coil locations with respect to three anatomical landmarks, i.e., the nasion and two preauricular points, were identified by using a 3D digitizer (FASTRAK™; Polhemus, Colchester, VT, USA). The obtained information was used to co-register the MRI, MEG, and head coordinates so that the MEG signal sources could be superimposed on the subject's MR image. The signals were recorded with a bandpass filter of 0.1-200 Hz and digitized at 600 Hz. MEG interictal spikes were inspected visually. Two epileptologists specialized in MEG had to reach a consensus before confirming an epileptogenic spike. The dipoles of these spikes were then localized using the single equivalent current dipole (ECD) method, implemented in the Neuromag software (Elekta Neuromag, Helsinki, Finland). Only ECDs with a goodness-of-fit value > 85% and a confidence volume < 3 µL were accepted. MEG ECDs were clustered using a hierarchical clustering method with the input of Euclidean distance between ECDs in each patient in SPSS 19.0 (17). In addition to the presurgical results, we also compared the MEG ECD clustered area with the surgically resected area as determined from a postoperative MR image.
We evaluated surgical outcome after a follow-up period of at least 1 yr. Surgical outcome was classified according to modified Engel criteria (10). Pathology was reviewed in all patients who underwent resection. Interpretation of the surgical specimens was completed by a neuropathologist. All patients had focal cortical dysplasia (FCD), except one who was found normal. We classified FCD according to Blumcke's criteria (7). There were 18 patients with FCD type IA, 2 patients with type IB, and 2 patient type IIA.
All analysis was performed by using SPSS 19.0 software (IBM, Armonk, NY, USA). Localization of individual presurgical tests was determined for hemispheric and lobar locations based on visual analysis. The localizing and lateralizing results from each electrophysiological modality were compared with the iEEG IOZ in order to calculate concordance rates. We used the chi-squared test or, where appropriate, Fisher's exact test. A value of P < 0.05 was considered statistically significant.
Postoperative seizure outcome was Engel class I in 5 (22%) patients, class II in 3 (13%), class III in 10 (43%), and class IV in 5 (22%) patients. Table 1 presents a summary of the patients' profiles.
Of the 23 patients, 9 had frontal lobe epilepsy (FLE), 11 had neocortical temporal lobe epilepsy (nTLE), 1 had parietal lobe epilepsy (PLE), 1 had occipital lobe epilepsy (OLE), and 1 had multifocal epilepsy (Table 2). The seizure-free rate for FLE patients (0/9) was less than that for nTLE (5/11) (P = 0.02, Table 2).
Compared to the iEEG-defined IOZ, the highest to the lowest hemispheric concordance rates among the electrophysiological modalities were MEG (83%) > ictal VEEG (78%) > PET (70%) > ictal SPECT (57%), and the highest to lowest lobar concordance rates were ictal VEEG = MEG (65%) > PET (57%) > ictal SPECT (52%). The MEG results showed the highest concordance rate with the IOZ at the hemispheric and lobar levels (Table 3). Moreover, the hemispheric concordance of the MEG results with the iEEG IOZ was significantly higher than that from ictal SPECT (P = 0.031). The relationship between hemispheric and lobar concordance rates and surgical outcome are presented in Table 3. We regarded patients with Engel class I or II as having a good outcome, while Engel class III or IV patients had a poor outcome. Among patients with a good surgical outcome, MEG results showed the highest concordance rate at both hemispheric (100%) and lobar (88%) levels. PET results had the lowest hemispheric concordance rate (50%), while ictal SPECT had the lowest lobar concordance rate (38%). However, no significant differences among the four modalities were found in the concordance rates for patients with a good outcome.
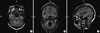
Table 4 presents the number of patients, stratified by surgical outcome, in which the presurgically identified ECDs were included in the final resection area. Fig. 1 shows the example of MEG ECDs superimposed on the post-operative MRI. Patients who underwent resection of the MEG ECDs had a significantly better surgical outcome (P = 0.038).
Table 5 presents the number of patients, by individual modality, in whom there was concordant and discordant localization of MEG ECDs at the hemispheric and lobar levels. None of the patients who showed MEG discordance with the iEEG IOZ, but in whom the IOZ was concordantly localized in the other presurgical evaluation modalities, had a good surgical outcome, with the exception of one patient for whom there was no concordant localization in any of presurgical examination modalities.
In this study, we determined concordance rates between the iEEG-defined IOZ and the localization results from four other presurgical modalities (SPECT, PET, VEEG, and MEG) in order to identify the relative value of individual modalities to surgical decisions made for adult nonlesional neocortical epilepsy patients. In addition, we elucidated the value of preoperative MEG interictal spike clusters in predicting surgical outcome in those patients. In our study, 23 adult epilepsy patients with no abnormal MRI findings, who had undergone surgical resection, and who had at least of 1 yr of follow-up were retrospectively analyzed. The MEG results showed the highest concordance with the iEEG IOZ at both hemispheric (83%) and lobar (65%) levels. In patients with a good surgical outcome, MEG also showed the highest concordance rate at both hemispheric (100%) and lobar (88%) levels. Our analysis of MEG ECD resection and surgical outcome showed that patients who had resection of MEG ECDs had a better surgical outcome. Furthermore, none of patients who showed MEG discordance, but in whom the IOZ was concordantly localized by other presurgical evaluation modalities, had good surgical outcome, except for one patient in whom concordant localization was not shown in any of the presurgical examinations.
Despite the importance of understanding intractable nonlesional epilepsy in patients whose seizure-free rates are limited to around 40% after surgery, studies concurrently comparing various presurgical evaluation tools are rare (6, 12, 18). Moreover, there is only one study that includes MEG in its comparison of epileptogenic focus-localizing sensitivity in various modalities (12). That study concluded that MEG and SPECT are better tools than PET for such localization; however, their patient group was limited to children. The present study is the first to compare the localizing value of various presurgical modalities (MEG, VEEG, SPECT, and PET) with iEEG in adult nonlesional epilepsy patients. In our comparison of those modalities with the iEEG IOZ, MEG had the highest concordance rate in both hemispheric (83%) and lobar (65%) levels. The hemispheric and lobar concordance rates of the other modalities were, respectively, 78% and 65% for ictal VEEG, 70% and 57% for PET, and 57% and 52% for ictal SPECT. In a multimodal comparison study of 89 nonlesional epilepsy patients, Lee et al. reported that ictal EEG (70.8%) had the highest sensitivity in locating the epileptogenic focus (6). Their concordance rate for PET and SPECT were 44.3% and 41.1%, respectively. Although MEG was not compared in their study, their concordance rates were similar to, or lower than, our rates.
In a study of interictal spike source localization, Brodbeck et al. evaluated 10 nonlesional epilepsy patients, and found a high correspondence (88.9%) between EEG interictal spike source localization and the iEEG IOZ (18). In their study, correspondences of interictal and ictal SPECT, and PET with the iEEG IOZ were 25%, 62.5%, and 55.6%, respectively. Although their source localization correspondence via EEG was greater than that from other presurgical modalities, EEG has an inherent signal conduction problem that makes accurate localization difficult. Compared to conventional EEG, MEG has advantages in localizing epileptogenic zones because MEG magnetic signals can pass through the human skull and other tissues without significant distortion (15). Previous studies have shown that MEG is particularly useful in the characterization of neocortical epilepsy (19-21).
Some studies have compared MEG with other presurgical evaluation tools in nonlesional epilepsy patients. Seo et al., in a comparison with iEEG, reported that MEG and SPECT had the highest concordance rates (79%, 11/14) while PET had a correspondence rate of 13% (3/14) (12). However, they included only 14 children in their patient group. Zhang et al. included 20 adult nonlesional epilepsy patients in their study (16), and reported MEG to have the highest concordance rate with iEEG (65%). However, they only compared two modalities (MEG and EEG) with iEEG. Garcia-Morales et al. explored the role of MEG in the localization of epileptiform activity in four patients with startle epilepsy and normal brain MRI results (13). They found MEG localizations were similar to the results of VEEG monitoring.
MEG concordance rates in our series are similar to those in recent reports, showing that localization via MEG is well matched with iEEG IOZ in nonlesional epilepsy. These results imply that MEG more useful than other modalities when planning epilepsy surgery.
Zhang et al. reported more favorable patient outcome when MEG findings are concordant with MRI or iEEG findings than when they are discordant (16). In our results, patients with a good surgical outcome had high concordance between MEG and iEEG IOZs at both hemispheric (100% vs 70%) and lobar (88% vs 53%) levels; however, the differences were not statistically significant. Since MEG had the highest localization value, we looked at the concordance in the other modalities when MEG results were concordant or discordant with iEEG IOZ. In our study, 4 patients displayed hemispheric discordance of MEG localization with the iEEG IOZ, 3 had concordant localization of an ictal onset hemisphere in ictal VEEG, and 3 had concordant localization in PET (Table 5). None of patients with MEG discordance but with concordant localization in the other presurgical evaluation modalities had a good surgical outcome. One good outcome patient did not have concordant localization in any of the presurgical examinations. Of the 8 lobe-discordant patients, ictal VEEG provided concordant localization in the iEEG IOZ lobe in 4 patients, ictal SPECT localization was concordant in 3 patients, and PET localization was concordant in 3 patients.
Although the concordance rate was highest in MEG, we found that other modalities may localize concordantly, when MEG provides inaccurate information. We conclude that multimodal approaches are desirable when attempting to improve the accuracy of epileptogenic foci localization in nonlesional epilepsy patients.
A study investigating the consequences of clustered MEG ECDs resection on surgical outcome suggested that there is tendency to have a seizure-free outcome in patients who had MEG ECDs in the final resection area (14). Although that study focused on children, in our series of adult patients, we observed similar results; i.e., patients who had resection of MEG ECDs had a better surgical outcome (P = 0.038) than those without such resection. As there are few studies on the effect of MEG ECD resection, especially in nonlesional adult patients, it is difficult to apply these results directly for clinical purposes. Based on our results, however, we suggest that using MEG localization information could improve success rate of that surgery when planning a resective surgery.
Researchers who have investigated the relationship between seizure onset lobe and surgical outcome agree that FLE patients generally have a poorer outcome than that in patients with other types of epilepsy (22-25). Our results support those findings as the surgical outcome for FLE in our study were significantly worse than that for nTLE or other types of epilepsy (P = 0.031). FLE is often characterized by a widespread epileptogenic zone, rapid propagation, wide areas of the frontal lobe, the presence of an eloquent cortex, and a variety of seizure semiologies. Due to these inherent impediments to the identification of epileptogenic foci, the delineation of the epileptogenic zone is more difficult, and surgical outcome is less successful in patients with nonlesional FLE compared to FLE patients with lesions (22, 23, 25). These FLE characteristics may have had an impact on surgical outcome in our FLE patients.
Histological examination revealed that all but one of the patients had FCD. There were 18 patients with FCD type IA, 2 with type IB, and 2 with type IIA. After hippocampal sclerosis and tumor, FCD is one of the most frequent pathologies in epilepsy surgery. The incidence of FCD in our series is similar to that in a previous report in which 91% (10/11) of the nonlesional epilepsy patients had type I FCD (12). In other reports, FCD type I was often associated with negative MRI results and a poor surgical outcome, while FCD type II was associated with visible MRI findings and a better outcome (26, 27). Several previous studies have found a high incidence of cortical dysplasia in patients with normal MRI findings who underwent surgery (6, 10, 25, 28-30). However, the high incidence of FCD in our nonlesional epilepsy patients is in line with those previous reports.
A major limitation of this study is a patient selection bias toward surgery, which is inherent in a retrospective study design at a single institute. As Lee et al. noted, because patients with localized ictal VEEG could have been recruited more easily for surgery, especially in the absence of a structural lesion, every presurgical modality showed a high concordance rate with the IOZ (6). Such high concordance rates may result in a lack of statistically significant differences among the different presurgical evaluation tools.
Another limitation of our study is that iEEG placement can be influenced by the results of presurgical tests. Thus, and as previously discussed by Seo et al., iEEG findings are not completely independent from the other presurgical tests (12). In addition, there is a possibility that iEEG placement may not cover a sufficient area to localize the epileptogenic foci, which may result in inaccurate foci localization. Nevertheless, this study has clinical relevance because we compared patients that were treated according to a current diagnostic paradigm.
In this study, we compared results from various presurgical evaluation modalities with iEEG-defined IOZs to elucidate the value of individual modalities to surgical decisions. By computing the concordance rate of each modality, we showed that MEG has the highest concordance with the iEEG IOZ. Furthermore, resection of MEG ECDs has prognostic significance in predicting surgical outcome. The present study is the largest study that concurrently compares various presurgical modalities in a nonlesional intractable adult epilepsy population. From the results, we conclude that MEG provides information that is important to decisions regarding surgical candidacy in intractable nonlesional epilepsy patients.
Figures and Tables
Fig. 1
MEG spike sources superimposed on the post-operative MRI in patient 4. Axial (A), coronal (B), and sagittal image (C) show MEG spike sources clustered around the surgically resected area. R, right; L, left; H, head; F, foot; A, anterior; P, posterior.
References
1. Berkovic SF, McIntosh AM, Kalnins RM, Jackson GD, Fabinyi GC, Brazenor GA, Bladin PF, Hopper JL. Preoperative MRI predicts outcome of temporal lobectomy: an actuarial analysis. Neurology. 1995. 45:1358–1363.
2. Tonini C, Beghi E, Berg AT, Bogliun G, Giordano L, Newton RW, Tetto A, Vitelli E, Vitezic D, Wiebe S. Predictors of epilepsy surgery outcome: a meta-analysis. Epilepsy Res. 2004. 62:75–87.
3. Yun CH, Lee SK, Lee SY, Kim KK, Jeong SW, Chung CK. Prognostic factors in neocortical epilepsy surgery: multivariate analysis. Epilepsia. 2006. 47:574–579.
4. Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B, Cavalcanti D, Baulac M. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998. 51:1256–1262.
5. Duncan JS. Imaging and epilepsy. Brain. 1997. 120:339–377.
6. Lee SK, Lee SY, Kim KK, Hong KS, Lee DS, Chung CK. Surgical outcome and prognostic factors of cryptogenic neocortical epilepsy. Ann Neurol. 2005. 58:525–532.
7. Holmes MD, Born DE, Kutsy RL, Wilensky AJ, Ojemann GA, Ojemann LM. Outcome after surgery in patients with refractory temporal lobe epilepsy and normal MRI. Seizure. 2000. 9:407–411.
8. Alarcon G, Valentin A, Watt C, Selway RP, Lacruz ME, Elwes RD, Jarosz JM, Honavar M, Brunhuber F, Mullatti N, et al. Is it worth pursuing surgery for epilepsy in patients with normal neuroimaging? J Neurol Neurosurg Psychiatry. 2006. 77:474–480.
9. Vinton AB, Carne R, Hicks RJ, Desmond PM, Kilpatrick C, Kaye AH, O'Brien TJ. The extent of resection of FDG-PET hypometabolism relates to outcome of temporal lobectomy. Brain. 2007. 130:548–560.
10. Chassoux F, Rodrigo S, Semah F, Beuvon F, Landre E, Devaux B, Turak B, Mellerio C, Meder JF, Roux FX, et al. FDG-PET improves surgical outcome in negative MRI Taylor-type focal cortical dysplasias. Neurology. 2010. 75:2168–2175.
11. von Oertzen TJ, Mormann F, Urbach H, Reichmann K, Koenig R, Clusmann H, Biersack HJ, Elger CE. Prospective use of subtraction ictal SPECT coregistered to MRI (SISCOM) in presurgical evaluation of epilepsy. Epilepsia. 2011. 52:2239–2248.
12. Seo JH, Holland K, Rose D, Rozhkov L, Fujiwara H, Byars A, Arthur T, DeGrauw T, Leach JL, Gelfand MJ, et al. Multimodality imaging in the surgical treatment of children with nonlesional epilepsy. Neurology. 2011. 76:41–48.
13. Garcia-Morales I, Maestu F, Perez-Jimenez MA, Elices E, Ortiz T, Alvarez-Linera J, Gil-Nagel A. A clinical and magnetoencephalography study of MRI-negative startle epilepsy. Epilepsy Behav. 2009. 16:166–171.
14. Ramachandrannair R, Otsubo H, Shroff MM, Ochi A, Weiss SK, Rutka JT, Snead OC 3rd. MEG predicts outcome following surgery for intractable epilepsy in children with normal or nonfocal MRI findings. Epilepsia. 2007. 48:149–157.
15. Funke ME, Moore K, Orrison WW Jr, Lewine JD. The role of magnetoencephalography in "nonlesional" epilepsy. Epilepsia. 2011. 52:10–14.
16. Zhang R, Wu T, Wang Y, Liu H, Zou Y, Liu W, Xiang J, Xiao C, Yang L, Fu Z. Interictal magnetoencephalographic findings related with surgical outcomes in lesional and nonlesional neocortical epilepsy. Seizure. 2011. 20:692–700.
17. Jeong W, Chung CK, Kim JS. MEG interictal spike clustering in relation with surgical outcome of cortical dysplasia. J Korean Neurosurg Soc. in press.
18. Brodbeck V, Spinelli L, Lascano AM, Pollo C, Schaller K, Vargas MI, Wissmeyer M, Michel CM, Seeck M. Electrical source imaging for presurgical focus localization in epilepsy patients with normal MRI. Epilepsia. 2010. 51:583–591.
19. Wheless JW, Willmore LJ, Breier JI, Kataki M, Smith JR, King DW, Meador KJ, Park YD, Loring DW, Clifton GL, et al. A comparison of magnetoencephalography, MRI, and V-EEG in patients evaluated for epilepsy surgery. Epilepsia. 1999. 40:931–941.
20. Stefan H, Hummel C, Hopfengartner R, Pauli E, Tilz C, Ganslandt O, Kober H, Moler A, Buchfelder M. Magnetoencephalography in extratemporal epilepsy. J Clin Neurophysiol. 2000. 17:190–200.
21. Moore KR, Funke ME, Constantino T, Katzman GL, Lewine JD. Magnetoencephalographically directed review of high-spatial-resolution surface-coil MR images improves lesion detection in patients with extratemporal epilepsy. Radiology. 2002. 225:880–887.
22. Moeller F, Tyvaert L, Nguyen DK, LeVan P, Bouthillier A, Kobayashi E, Tampieri D, Dubeau F, Gotman J. EEG-fMRI: adding to standard evaluations of patients with nonlesional frontal lobe epilepsy. Neurology. 2009. 73:2023–2030.
23. Lee JJ, Lee SK, Lee SY, Park KI, Kim DW, Lee DS, Chung CK, Nam HW. Frontal lobe epilepsy: clinical characteristics, surgical outcomes and diagnostic modalities. Seizure. 2008. 17:514–523.
24. Kellinghaus C, Luders HO. Frontal lobe epilepsy. Epileptic Disord. 2004. 6:223–239.
25. Jeha LE, Najm I, Bingaman W, Dinner D, Widdess-Walsh P, Luders H. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain. 2007. 130:574–584.
26. Chung CK, Lee SK, Kim KJ. Surgical outcome of epilepsy caused by cortical dysplasia. Epilepsia. 2005. 46:Suppl 1. 25–29.
27. Krsek P, Maton B, Korman B, Pacheco-Jacome E, Jayakar P, Dunoyer C, Rey G, Morrison G, Ragheb J, Vinters HV, et al. Different features of histopathological subtypes of pediatric focal cortical dysplasia. Ann Neurol. 2008. 63:758–769.
28. Nobili L, Francione S, Mai R, Cardinale F, Castana L, Tassi L, Sartori I, Didato G, Citterio A, Colombo N, et al. Surgical treatment of drug-resistant nocturnal frontal lobe epilepsy. Brain. 2007. 130:561–573.
29. Hong KS, Lee SK, Kim JY, Lee DS, Chung CK. Pre-surgical evaluation and surgical outcome of 41 patients with non-lesional neocortical epilepsy. Seizure. 2002. 11:184–192.
30. McGonigal A, Bartolomei F, Regis J, Guye M, Gavaret M, Trebuchon-Da Fonseca A, Dufour H, Figarella-Branger D, Girard N, Peragut JC, et al. Stereoelectroencephalography in presurgical assessment of MRI-negative epilepsy. Brain. 2007. 130:3169–3183.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download