Abstract
High-mobility group box 1 (HMGB1) protein has been demonstrated to play an important role in chronic inflammatory diseases including rheumatoid arthritis, and systemic lupus erythematosus. This study investigated the association between extracellular HMGB1 expression and disease activity, and clinical features of Behçet's disease (BD). Extracellular HMGB1 expression in the sera of 42 BD patients was measured and was compared to that of 22 age- and sex-matched healthy controls. HMGB1 expression was significantly increased in BD patients compared to healthy controls (78.70 ± 20.22 vs 10.79 ± 1.90 ng/mL, P = 0.002). In addition, HMGB1 expression was significantly elevated in BD patients with intestinal involvement compared to those without (179.61 ± 67.95 vs 61.89 ± 19.81 ng/mL, P = 0.04). No significant association was observed between HMGB1 concentration and other clinical manifestations, or disease activity. It is suggested that extracellular HMGB1 may play an important role in the pathogenesis of BD.
Behçet's disease (BD) is a chronic multisystemic inflammatory disorder of unknown etiology, consisting of orogenital ulceration, ocular inflammation, intestinal involvement and skin lesions (1, 2). The innate immune system is involved in BD pathogenesis as evidenced by frequent mucocutaneous symptoms, Toll-like receptor (TLR) expression in affected cells, and neutrophil hyper-responsiveness to Streptococcus sanguis antigens and heat shock proteins (HSPs) (1, 3, 4).
High-mobility group box 1 (HMGB1) protein functions to stabilize nucleosome formation and also acts as a transcription-factor-like protein. It is an important damage-associated molecular pattern molecules (DAMPs), along with HSPs and S100 protein (5). HMGB1 is a crucial cytokine that mediates the response to infection and inflammation. It activates macrophages and monocytes to release proinflammatory cytokines, upregulates endothelial adhesion molecules, and stimulates epithelial cell barrier failure (5, 6). HMGB1 is released from necrotic cells and secreted from activated macrophages, dendritic cells, and natural killer cells. It has an important function in chronic inflammatory diseases including rheumatoid arthritis, systemic lupus erythematosus, and inflammatory myositis (6-11).
Innate immunity has been investigated as a critical step in initiating and aggravating inflammation in BD. HMGB1 is known as a potent stimulator of the innate immune system and mediates the immune process between innate and adaptive immunity (12). The expression of HMGB1 and its affect on BD, however, are currently not known. Thus, the aim of this study was to investigate the extracellular HMGB1 expression in BD, and the association between extracellular HMGB1 expression and disease activity, and clinical features of BD.
The experimental protocols used in this study were approved by the local ethics committee and informed consent was obtained from each patient. Forty-two patients with BD fulfilled the diagnostic criteria of the International BD Study Group (13). The control group consisted of 22 healthy age- and sex-matched volunteers with no history of autoimmune diseases or significant severe infection at the time of sampling. For all study patients enrolled, the following demographic and clinical data were obtained from medical records: age at the time of diagnosis of BD, sex, duration of disease, clinical signs and symptoms, and laboratory data including neutrophil count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). Both clinical and laboratory findings were used to classify active and inactive BD patients. Active disease patients were defined as those with worsening clinical symptoms at the time of study visit in addition to at least three of the following major findings: oral ulcerations or stomatitis; genital ulcers; anterior iridocyclitis, posterior vasculitis or panuveitis; cutaneous findings; or positive pathergy test (14). Ethylenediaminetetraacetate serum samples of BD patients and healthy controls were centrifuged at 1,500 g for 10 min and the supernatants were stored at -20℃ until use. HMGB1 levels in the serum were quantified using a commercially available ELISA Kit, according to the manufacturer's instructions (IBL international GMBH, Hamburg, Germany). In statistical analysis, data are expressed as means and standard error of mean (SEM) and as percentages, unless otherwise indicated. The normality of data was tested by Shapiro-Wilk test. To compare continuous variable distributions and categorical variables between groups, we used Student's t-test or Mann-Whitney U test and the chi-squared-test or Fisher's Exact test, respectively. Correlations between groups were determined using Spearman's rank correlation analysis. Differences were considered significant at P < 0.05 level. The PASW statistics software package (version 17.0, SPSS Institute, Chicago, IL, USA) was used for all analyses.
The 42 BD patients contained 25 active and 17 inactive patients with a mean age of 47 yr (range 22-65 yr). The 22 healthy controls were 10 males and 12 females with a mean age of 43 yr (range 28-67 yr). No differences were seen in gender and age between the 42 patients and the 22 healthy controls. In BD group, skin lesions (81.0%), including erythema nodosum, were the second most common clinical finding, followed by genital ulceration (73.8%), arthritis (45.2%), vascular involvement (28.6%), ocular lesions (19%), and intestinal lesions (14.3%). No patients showed central nervous system involvement.
As shown in Fig. 1A, the extracellular HMGB1 concentration in serum samples of BD patients and healthy controls as measured by ELISA were 78.70 ± 20.22 ng/mL and 10.79 ± 1.90 ng/mL, respectively (P = 0.002). Next, we measured extracellular HMGB1 expression in the serum of 25 active and 17 inactive patients. The extracellular HMGB1 expression was higher in active patients than inactive patients (92.26 ± 31.63 vs 58.78 ± 18.46 ng/mL), although this difference did not reach statistical significance (P = 0.367, Fig. 1B).
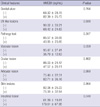
We evaluated the difference in extracellular HMGB1 expression by the presence of clinical features in BD patients (Table 1). Patients with intestinal involvement had a higher mean extracellular HMGB1 expression than those without intestinal symptom (179.61 ± 67.95 vs 61.89 ± 19.81 ng/mL, P = 0.04). Patients with vascular involvement showed a trend toward decreased serum HMGB1 expression compared to those without, but did not reach statistical significance (36.79 ± 12.63 vs 95.47 ± 27.41 ng/mL, P = 0.059). No significant association was observed between extracellular HMGB1 concentration and other clinical manifestations, including genital ulcer, erythema nodosum, ocular involvement or musculoskeletal symptoms. No significant correlation was found between HMGB1 concentration and leukocyte counts, ESR, or CRP (r = -0.248, P = 0.113; r = -0.010, P = 0.951; r = -0.104, P = 0.514).
The innate immune system and neutrophil hyper-responsiveness are involved in BD pathogenesis (1, 3, 4). HMGB1 is a well-characterized DAMP, like the defensins, and functions in the innate immune system. As a cytokine, HMGB1 activates endothelial cell, promotes angiogenesis, enhances hematopoietic stem-cell migration, and initiates inflammation (5). We found that the mean extracellular HMGB1 expression is increased seven-to-eight-fold in BD patients compared to healthy controls. Extracellular HMGB1 enhances the expression of pro-inflammatory cytokines such as TNF-α, IL-6 and IFN-γ through the NF-κB pathway in human neutrophils (15), and neutrophils are critical for mediating BD development. Many studies found an increase in pro-inflammatory cytokines such as IL-1, IL-8, IL-12, IL-15, and TNF-α in the sera of BD patients (16). Considering these findings and our results, these pro-inflammatory effects of extracellular HMGB1 are likely to be involved in various clinical manifestations of BD.
We demonstrated that HMGB1 concentration was significantly elevated in patients with intestinal involvement compared to those without intestinal involvement. Sappington et al. reported that both HMGB1 and B box proteins increased the permeability of human enterocytic cell lines in a time- and concentration-dependent manner. Administration of HMGB1 B box to wild-type mice increases ileal mucosal permeability, and HMGB1 or B box induces increased nitric oxide (NO) production and increased induction of NO synthase mRNA (17). Extracellular HMGB1 increased production of TNF-α and IFN-γ (5, 15, 18). Thus, extracellular HMGB1 may influence development and aggravation of intestinal lesion of BD through increased NO and pro-inflammatory cytokine production and induction of NO synthase expression.
HMGB1 activates endothelial cells, leading to increased expression of adhesion molecules such as intracellular adhesion molecule 1 and vascular cell-adhesion molecule 1 (19). This raises the possibility that HMGB1 is involved in the vascular injury of BD. Orogenital ulcerations, and erythema-nodosum-like lesions are examples of direct injury to the vessel wall. However, no differences in extracellular HMGB1 levels were seen with genital ulcerations, erythema nodosum-like lesion, or vascular involvement. Unexpectedly, although this finding was not significant, patients without vascular involvement showed higher levels of extracellular HMGB1 compared to those with this symptom. The number of investigated patients with BD might be small for this conclusive result. Therefore, the role of extracellular HMGB1 in vascular manifestation of BD warrants further investigation.
No serological marker in assessing disease activity is available in BD. Many researchers have attempted to identify specific serologic markers to predict disease activity or prognosis. Recently, it has been reported that a soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) in BD patients with intestinal involvement was significantly higher than those in healthy controls, suggesting possible role of serum sTREM-1 level as a potential marker for disease activity of intestinal BD (20). The associations of HMGB1 level with intestinal involvement and possibly vascular involvement in the present study further suggest that HMGB1 may be utilized as a valuable serological marker of the severity of inflammation. Further studies will focus on the role of extracellular HMGB1 as the inflammatory marker potentially reflecting disease activity of BD.
This is the first study to evaluate the expression of HMGB1 in BD. An important finding is that extracellular HMGB1 expression is significantly increased in BD patients compared to healthy controls, and is significantly increased in the sera of BD patients with intestinal involvement compared to those without intestinal involvement. It is suggested that extracellular HMGB1 may play an important role in the pathogenesis of BD. Further studies are needed to elucidate the extracellular HMGB 1 as a potential therapeutic target in the treatment of intestinal BD.
Figures and Tables
References
1. Sakane T, Takeno M, Suzuki N, Inaba G. Behçet's disease. N Engl J Med. 1999. 341:1284–1291.
2. Yi SW, Cheon JH, Kim JH, Lee SK, Kim TI, Lee YC, Kim WH. The prevalence and clinical characteristics of esophageal involvement in patients with Behçet's disease: a single center experience in Korea. J Korean Med Sci. 2009. 24:52–56.
3. Direskeneli H. Behçet's disease: infectious aetiology, new autoantigens, and HLA-B51. Ann Rheum Dis. 2001. 60:996–1002.
4. Yazici H, Fresko I, Yurdakul S. Behçet's syndrome: disease manifestations, management, and advances in treatment. Nat Clin Pract Rheumatol. 2007. 3:148–155.
5. Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005. 5:331–342.
6. Andersson U, Harris HE. The role of HMGB1 in the pathogenesis of rheumatic disease. Biochim Biophys Acta. 2010. 1799:141–148.
7. Kokkola R, Sundberg E, Ulfgren AK, Palmblad K, Li J, Wang H, Ulloa L, Yang H, Yan XJ, Furie R, Chiorazzi N, Tracey KJ, Andersson U, Harris HE. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum. 2002. 46:2598–2603.
8. Taniguchi N, Kawahara K, Yone K, Hashiguchi T, Yamakuchi M, Goto M, Inoue K, Yamada S, Ijiri K, Matsunaga S, Nakajima T, Komiya S, Maruyama I. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003. 48:971–981.
9. Ulfgren AK, Grundtman C, Borg K, Alexanderson H, Andersson U, Harris HE, Lundberg IE. Down-regulation of the aberrant expression of the inflammation mediator high mobility group box chromosomal protein 1 in muscle tissue of patients with polymyositis and dermatomyositis treated with corticosteroids. Arthritis Rheum. 2004. 50:1586–1594.
10. Jiang W, Pisetsky DS. Expression of high mobility group protein 1 in the sera of patients and mice with systemic lupus erythematosus. Ann Rheum Dis. 2008. 67:727–728.
11. Abdulahad DA, Westra J, Limburg PC, Kallenberg CG, Bijl M. HMGB1 in systemic lupus Erythematosus: its role in cutaneous lesions development. Autoimmun Rev. 2010. 9:661–665.
12. Pisetsky DS, Erlandsson-Harris H, Andersson U. High-mobility group box protein 1 (HMGB1): an alarmin mediating the pathogenesis of rheumatic disease. Arthritis Res Ther. 2008. 10:209.
13. International Study Group for Behçet's Disease. Criteria for diagnosis of Behçet's disease. International Study Group for Behçet's Disease. Lancet. 1990. 335:1078–1080.
14. Turkoz Y, Evereklioglu C, Ozkiris A, Mistik S, Borlu M, Ozerol IH, Duygulu F, Ilhan O. Serum levels of soluble P-selectin are increased and associated with disease activity in patients with Behçet's syndrome. Mediators Inflamm. 2005. 2005:237–241.
15. Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ, Abraham E. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol. 2003. 284:C870–C879.
16. Zouboulis CC, May T. Pathogenesis of Adamantiades-Behçet's disease. Adv Exp Med Biol. 2003. 528:161–171.
17. Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002. 123:790–802.
18. Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000. 192:565–570.
19. Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, Morser J, Arnold B, Preissner KT, Nawroth PP. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003. 198:1507–1515.
20. Jung YS, Kim SW, Yoon JY, Lee JH, Jeon SM, Hong SP, Kim TI, Kim WH, Cheon JH. Expression of a soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) correlates with clinical disease activity in intestinal Behçet's disease. Inflamm Bowel Dis. 2011. doi: 10.1002/ibd.21600.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download