Abstract
Laribacter hongkongensis is an emerging pathogen in patients with community-acquired gastroenteritis and traveler's diarrhea. We herein report a case of L. hongkongensis infection in a 24-yr-old male with liver cirrhosis complicated by Wilson's disease. He was admitted to a hospital with only abdominal distension. On day 6 following admission, he complained of abdominal pain and his body temperature reached 38.6℃. The results of peritoneal fluid evaluation revealed a leukocyte count of 1,180/µL (polymorphonuclear leukocyte 74%). Growth on blood culture was identified as a gram-negative bacillus. The isolate was initially identified as Acinetobacter lwoffii by conventional identification methods in the clinical microbiology laboratory, but was later identified as L. hongkongensis on the basis of molecular identification. The patient was successfully treated with cefotaxime. To the best of our knowledge, this case is the first report of hospital-acquired L. hongkongensis bacteremia with neutrophilic ascites.
Laribacter hongkongensis is a facultative anaerobic, motile, non-sporulating gram-negative bacillus. It belongs to the family Neisseriaceae of the β subclass of Proteobacteria (1). Since the first description of L. hongkongensis from the blood and empyema pus of a patient with alcoholic liver cirrhosis in 2001, the bacterium has subsequently been associated with community-acquired gastroenteritis (1, 2). However, accurate identification of L. hongkongensis is impossible using commercially-available phenotypic identification systems. Thus, the prevalence, pathogenic potential, and epidemiology of L. hongkongensis are unclear. The identification based on a molecular approach could permit more accurate determinations of incidence and evaluation of clinical relevance.
Although L. hongkongensis has been reported in Hong Kong, China, and Hungary (3), it has not hitherto been identified in Korea. Recently, we experienced a case of L. hongkongensis bacteremia with neutrophilic ascites. The bacterium was originally misidentified as Acinetobacter lwoffii. Proper identification was achieved using a molecular approach.
A 24-yr-old male was admitted to a hospital, on August 16, 2007, with a 15-day history of abdominal distension. The patient had been diagnosed with Wilson's disease at 17-yr-of-age and had since undergone treatment for liver cirrhosis complicated by Wilson's disease. The patient had no history of overseas travel and had no recall of ingesting undercooked freshwater fish for at least the previous year. On physical examination, blood pressure was 98/49 mmHg, pulse rate was 96 beats per min, and body temperature (36.5℃). All measurements were within the normal range. Abdominal examination revealed a diffusely distended abdomen, diminished bowel sound, and splenomegaly. Laboratory tests revealed a hemoglobin concentration of 8.3 g/dL, leukocyte count of 6,248/µL, platelet count of 42,000/µL, serum creatinine level of 0.94 mg/dL, serum bilirubin level of 6.7 mg/dL, and serum albumin level of 1.6 g/dL. The analysis of peritoneal fluid demonstrated an albumin level of 202 mg/dL and leukocyte count of 130/µL (poly 15%). Dose adjustment of diuretics was done to control ascites. The patient's subsequent hospital course was uneventful. But, on day 6 following admission, fever developed, with a body temperature reaching 38.6℃. Examination showed diffuse tenderness of the entire abdomen. The results of peritoneal fluid evaluation revealed a leukocyte count of 1,180/µL (poly 74%). Empirical treatment with cefotaxime (2 g every 8 hr) was commenced with a presumptive diagnosis of spontaneous bacterial peritonitis. Two days later, growth on blood culture was identified as a gram-negative bacillus using the BacT ALERT 3D culture system. The isolate was designated 07AC-292. However, no organism was identified in the peritoneal fluid. The patient responded to cefotaxime and was discharged on post-admission day 12.
To identify isolate 07AC-292, biochemical characterization by VITEK2 with an ID-GNB card was performed in accordance with the manufacturer's instructions. The isolate was identified as A. lwoffii at a confidence level of 94%. However, an Acinetobacter differentiation method using the rpoB gene (4) indicated that the isolate did not belong to the genus Acinetobacter. Thus, we tried to identify isolate 07AC-292 accurately using a molecular method. The DNA of the isolate was extracted and a portion of the 16S rRNA gene was amplified and sequenced using the primer set as previously described (5). A 1,346-bp sequence was obtained. The determined sequences were compared with the GenBank public database using the BLASTn program (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The 16S rRNA sequence of the isolate 07AC-292 showed complete identity with several L. hongkongensis strains in the database. Thus, isolate 07AC-292 was definitively identified as L. hongkongensis. A phylogenetic tree constructed based on 16S rRNA gene sequences also supported the identity of isolate 07AC-292 as L. hongkongensis (Fig. 1).
Antibiotic susceptibility testing (ampicillin-sulbactam, cefepime, ceftriaxone, ceftazidime, amikacin, cefoperazone-sulbactam, imipenem, meropenem, doripenem, tetracycline, ciprofloxacin, rifampin, piperacillin-tazobactam, colistin, polymyxin B, and tigecycline) was conducted using the broth microdilution method according to Clinical and Laboratory Standards Institute (CLSI) protocol (6). Minimum inhibitory concentration breakpoints and quality-control protocols were used according to non-fermenting gram-negative organism guidelines established by the CLSI. Isolate 07AC-292 was resistant to piperacillin, intermediate resistant to ceftriaxon, and susceptible to the other tested antibiotics (Table 1).
L. hongkongensis was first isolated from a 54-yr-old Chinese male with alcoholic cirrhosis and thoracic bacateremic empyema (1, 2). Subsequently, the bacterium has been isolated from patients in other parts of the world. The isolation of L. hongkogensis from patients who are residents in, or recent travelers to, Asia, Europe, America, and Africa imply the global importance of the bacterium (7). Most reported cases were associated with recent travel and eating fish and symptoms are similar to salmonella and campylobacter gastroenteritis (2). Although freshwater fish is probably a major reservoir of L. hongkongensis, differences exist between isolates obtained from humans and fish using pulsed-field gel electrophoresis, suggesting that not all environmental clones are virulent (8).
The presently-reported patient, who had underlying liver cirrhosis associated with Wilson's disease, was suffering from neutrophilic ascites caused by L. hongkongensis. In contrast to previous described cases, the patient denied a history of ingestion of undercooked freshwater fish and overseas travel. It may be that the infection was hospital-acquired. Previous studies reported that this bacterium was associated with community-acquired gastroenteritis and traveler's diarrhea (2). It has been suggested that there was no carriage state of L. hongkogenesis (8), although the gastrointestinal tract has been suggested as a possible primary site of infection and enteric bacteria could enter the systemic circulation from the portal vein by passage through the liver in patients with portal hypertension (1, 2). The present L. hongkongensis infection may have originated from the intestinal flora. If so, it indicates that L. hongkongensis may exist in a carriage state in his intestine flora. In someone suffering from liver cirrhosis, the result could be a debilitating infection.
To date, all L. hongkongensis strains tested have been resistant to ampicillin and cephalosporins, but susceptible to the carbapenems, amoxicillin-clavulanate, aminoglycosides and fluoroquinolones (9). The isolate in this study showed a somewhat different antibiotic susceptibility profile, in that it was susceptible to ampicillin-sulbactam and several cephalosporins. A comparative study among L. hongkongensis isolates could more precisely determine the difference antibiotic susceptibility profiles, indicative of different origins.
To our knowledge, this case is the first report of hospital-acquired L. hongkongensis bacteremia with neutrophilic ascites. So far, no rapid and accurate commercial phenotypic identification exists for L. hongkongensis. Thus, L. hongkongensis could be frequently misidentified as other species. Isolate 07AC-292 was initially misidentified as A. lwoffii. Such misidentification might occur because L. honkingensis is not included in the database of commercial identification systems and biochemical tests are not enough to differentiate it from other species. Thus, it is conceivable that more L. hongkongensis infections have occurred, but have not been accurately identified due to the lack of proper identification tools. Since multidrug-resistant Acinetobacter sp. isolates are increasing in Korea and L. hongkongensis is susceptible to most antimicrobial agents, correct identification of L. hongkongensis may reduce the inappropriate use of antimicrobial agents. Further clinical experiences with invasive diseases caused by L. hongkongensis are necessary to evaluate its potential pathogenesis. Extensive epidemiologic studies should be carried out to ascertain the etiologic association between L. hongkongensis and the intestinal normal flora, and to identify the host and the routes of transmission of L. hongkongensis.
Figures and Tables
Fig. 1
Phylogenetic relationships of 07AC-292 and other species of the most similar sequences, retrieved from GenBank, based on partial 16S rRNA gene sequences. This unrooted tree was constructed by method of neighbor-joining. Numbers at branching nodes are percentages of 1,000 bootstrap replications.

Table 1
Minimum inhibitory concentrations (MICs) of the isolate 07AC-292 in the antimicrobial susceptibility testing
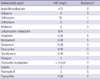
*Since no validated interpretative criteria are available for L. hongkongnsis, the CLSI breakpoints for Acinetobacter spp. were used to determine susceptibilities (6). R, resistant; I, intermediate; S, susceptible.
References
1. Yuen KY, Woo PC, Teng JL, Leung KW, Wong MK, Lau SK. Laribacter hongkongensis gen. nov., sp. nov., a novel gram-negative bacterium isolated from a cirrhotic patient with bacteremia and empyema. J Clin Microbiol. 2001. 39:4227–4232.
2. Woo PC, Lau SK, Teng JL, Que TL, Yung RW, Luk WK, Lai RW, Hui WT, Wong SS, Yau HH, Yuen KY. L. Hongkongensis study group. Association of Laribacter hongkongensis in community-acquired gastroenteritis with travel and eating fish: a multicentre case-control study. Lancet. 2004. 363:1941–1947.
3. Woo PC, Lau SK, Tse H, Teng JL, Curreem SO, Tsang AK, Fan RY, Wong GK, Huang Y, Loman NJ, Snyder LA, Cai JJ, Huang JD, Mak W, Pallen MJ, Lok S, Yuen KY. The complete genome and proteome of Laribacter hongkongensis reveal potential mechanisms for adaptations to different temperatures and habitats. PLoS Genet. 2009. 5:e1000416.
4. Ko KS, Suh JY, Kwon KT, Jung SI, Park KH, Kang CI, Chung DR, Peck KR, Song JH. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J Antimicrob Chemother. 2007. 60:1163–1167.
5. Heo ST, Ko KS, Kwon KT, Ryu SY, Bae IG, Oh WS, Song JH, Peck KR. The first case of catheter-related bloodstream infection caused by Nocardia farcinica. J Korean Med Sci. 2010. 25:1665–1668.
6. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing: 19th informational supplement. 2008. Wayne, Pa: Clinical and Laboratory Standards Institute;M100-S18.
7. Woo PC, Lau SK, Teng JL, Yuen KY. Current status and future directions for Laribacter hongkongensis, a novel bacterium associated with gastroenteritis and traveller's diarrhoea. Curr Opin Infect Dis. 2005. 18:413–419.
8. Teng JL, Woo PC, Ma SS, Sit TH, Ng LT, Hui WT, Lau SK, Yuen KY. Ecoepidemiology of Laribacter hongkongensis, a novel bacterium associated with gastroenteritis. J Clin Microbiol. 2005. 43:919–922.
9. Lau SK, Wong GK, Poon RW, Lee LC, Leung KW, Tse CW, Ho PL, Que TL, Woo PC, Yuen KY. Susceptibility patterns of clinical and fish isolates of Laribacter hongkongensis: comparison of the Etest, disc diffusion and broth microdilution methods. J Antimicrob Chemother. 2009. 63:704–708.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download