Abstract
Hyperoxic ventilation induces detrimental effects on the respiratory system, and ambient oxygen may be harmful unless compensated by physiological anti-oxidants, such as vitamin C. Here we investigate the changes in electrolyte transport of airway epithelium in mice exposed to normobaric hyperoxia and in gulonolacton oxidase knock-out (gulo[-/-]) mice without vitamin C (Vit-C) supplementation. Short-circuit current (Isc) of tracheal epithelium was measured using Ussing chamber technique. After confirming amiloride-sensitive Na+ absorption (ΔIsc,amil), cAMP-dependent Cl- secretion (ΔIsc,forsk) was induced by forskolin. To evaluate Ca2+-dependent Cl- secretion, ATP was applied to the luminal side (ΔIsc,ATP). In mice exposed to 98% PO2 for 36 hr, ΔIsc,forsk decreased, ΔIsc,amil and ΔIsc,ATP was not affected. In gulo(-/-) mice, both ΔIsc,forsk and ΔIsc,ATP decreased from three weeks after Vit-C deprivation, while both were unchanged with Vit-C supplementation. At the fourth week, tissue resistance and all electrolyte transport activities were decreased. An immunofluorescence study showed that the expression of cystic fibrosis conductance regulator (CFTR) was decreased in gulo(-/-) mice, whereas the expression of KCNQ1 K+ channel was preserved. Taken together, the CFTR-mediated Cl- secretion of airway epithelium is susceptible to oxidative stress, which suggests that supplementation of the antioxidant might be beneficial for the maintenance of airway surface liquid.
Hyperoxic inhalation (PO2 higher than that in the ambient air) is used in a variety of clinical practices including anaesthesia and post-operative recovery. The duration of hyperoxic ventilation can be prolonged depending on the patient conditions such as peripheral O2 saturation. Also, in patients with respiratory disorders which cause hypoxemia, long-term oxygen therapy is often used. Although the benefits of O2 supplementation are obvious in clinical situations of fatal hypoxemia, there also are harmful effects of O2. For example, the side effects of increasing concentrations of O2 supplementation are frequently observed as pulmonary injury (1). In neonatal neural tissues such as the retina, the harmful influence of hyperoxic ventilation has also been observed (2). The cough reflex, a defensive respiratory reflex, is also impaired in hyperoxia, and the inhibition of cough reflex is prevented by dietary antioxidants (3).
It is generally thought that tissue injury caused by O2 is mediated by the formation of reactive oxygen species (ROS), which can react with and damage essential biomolecules via lipid peroxidation, protein sulfhydryl oxidation, and DNA damage (4). Because airway epithelium is constantly and frequently exposed to oxidative stress, it is highly likely that ROS-mediated oxidative stress affects the functions of airway epithelium (5-7). In this context, there is increasing evidence for the protective effects of antioxidant supplementation in respiratory diseases (3, 4, 8).
Because humans cannot synthesize ascorbic acid, dietary uptake of vitamin C is essential to cope with oxidative stress and to preserve physiological homeostasis. It has been reported that vitamin C is present in airway surface liquid (ASL), a thin (10-30 µm) layer of fluid covering the luminal surface of the airway epithelium (9-11). The physiological role of vitamin C in ASL is to stimulate Cl- secretion via cAMP-activated Cl- channels known as cystic fibrosis transmembrane transport regulator (CFTR) in the luminal membrane of the airway epithelium (12). A balanced level of ASL is critical for the protection of the airway epithelium. For transepithelial fluid secretion, an electrogenic Cl- secretion model is regarded as the ionic mechanism in which the cAMP-dependent activations of the luminal Cl- channel (CFTR) and the basolateral K+ channel (potassium voltage-gated channel, KQT-like subfamily, member 1, KCNQ1) are critical steps (11, 13).
While there have been numerous studies on the structural and biochemical changes in respiratory epithelial cells in response to oxidative stress, a direct investigation on the physiological function (i.e., electrolyte secretion) has been rarely conducted. The studies by Cowley and Linsdell showed that exogenous hydrogen peroxide (H2O2, 0.5-2 mM) directly activates the electrogenic Cl- secretion of Calu-3, a cell line model of serous airway epithelial cells (14). In contrast, the oxidative stress caused by pyocyanin, a redox-active phenazine compound, impairs CFTR-dependent Cl- secretion in the bronchial epithelium (15). Consistent with this, vitamin C activates CFTR in primary cultured human airway epithelial cells (12).
Apart from the acute effects, chronic effects of oxidative stress and of vitamin C deprivation on the airway electrolyte transport are very important. To the best of our knowledge, there has been no investigation on the effects of sustained hyperoxic ventilation on the electrolyte secretion of airway epithelium in vivo. Also, the functional changes in the airway epithelium in the vitamin C-deficient animal model have not yet been investigated.
Unlike humans, rodents synthesize vitamin C (ascorbic acid) from glucose in situ. Recently, a mouse line has been generated with a targeted deletion of the gene coding for L-gulono-c-lactone oxidase (Gulo), which catalyzes the final step of ascorbic acid biosynthesis (16). Mice null for Gulo (gulo[-/-] mice) and that are not provided with dietary vitamin C supplements become scorbutic, lose weight, and eventually die. The ambient level of oxygen might induce oxidative stress when the intrinsic antioxidant levels are insufficient. In this respect, it was tempting to investigate the airway epithelial secretory function in gulo(-/-) mice exposed to normal atmospheric conditions.
Based on the results outlined above, we investigated electrolyte secretion and the absorption functions of mouse airway epithelium using the Ussing chamber apparatus to measure short-circuit current (Isc). The changes in Isc (ΔIsc) reflecting the Cl- secretion and Na+ absorption functions of airway epithelia were compared to mice exposed to hyperoxic conditions (80%-98% PO2) and to a normoxic environment. Also, we compared the Isc values in the airway epithelium of gulo(-/-) mice according to the duration of vitamin C deficiency.
ICR mice, C57BL/6 wild-type mice and gulo(-/-) mice were maintained in a specific pathogen-free condition in the animal facility at Seoul National University College of Medicine. Gulo(-/-) mice were maintained with or without 3.3 g/L of vitamin C supplementation in the drinking water. The effects of chronic hyperoxia were tested in ICR mice (Fig. 1). For chronic exposure to hyperoxic conditions (80%-98%, PO2), mice were kept in a semi-tight transparent chamber for 24-28 hr with automatic regulation of PO2 (Biospherix, Lacona, NY, USA). Control mice were kept in the same cage but with a half-open door, i.e., ambient air exposure. For the investigation of airway epithelial function in gulo(-/-) mice and the immunohistochemistry study, C57BL/6 mice were used as the control group.
Mice of both genders (body weight, 25-35 g) were sacrificed via inhalation of 100% CO2. The trachea was split along the anterior side, and the pars membranacea of the trachea was mounted into a tissue holder in the Ussing chamber (circular exposed area, 0.64 mm2) with the aid of a dissection microscope. The chamber (2 mL) was maintained at 37℃ and continuously perfused with a normal Tyrode's (NT) solution containing (in mM) 145 NaCl, 0.4 KH2PO4, 1.6 K2HPO4, 5 HEPES, 5 D-glucose, 1 MgCl2, 1.3 CaCl2 (pH 7.4) on both sides at a flow rate of 10-15 mL/min (For more detail of the instrument, please see supplementary photos in online-only material). Indomethacin (1 µM) was included in all experimental solutions to inhibit the endogenous formation of prostaglandins. The tissue was allowed to equilibrate for at least 30 min prior to the experiments. Transepithelial resistance (Rte) was determined from the voltage deflection (ΔVte) caused by the injection of current (Iinj, 0.8 µA, 1.4 sec of duration, 0.7 Hz) according to Ohm's law (Rte = ΔVte/Iinj). The resistance of the empty chamber was subtracted. The equivalent short circuit current (Isc) was calculated based on the trans-epithelial voltage (Vte) and Rte according to Ohm's law (Isc = Vte/Rte). The electrical signs of Isc and Vte refer to the luminal side. Amiloride, indomethacin, forskolin, and 293B were initially dissolved in dimethylsulfoxide (DMSO) and diluted with NT solution. The final concentration of DMSO was < 0.1%. All the chemicals used in the Ussing chamber study were purchased from Sigma-Korea (Seoul, Korea).
Mice were perfused with heparinized PBS, and the main trachea tissues were fixed in 4% paraformaldehyde at 4℃. Frozen sections with 5 µm thicknesses were post-fixed, and non-specific signals were blocked with 5% normal serum. Cut tissues were incubated with anti-CFTR antibody or anti-KCNQ1 antibody (Abcam, Cambridge, MA, USA) at 4℃ overnight in a humidified chamber and then incubated with AlexaFluor 555-conjugated secondary antibody (Invitrogen, Camarillo, CA, USA) for 1 hr at room temperature. Nuclei were counterstained with 4',6-diamidino-2-phenylindole (DAPI) and observed with confocal fluorescence microscopy (IX-81, Olympus, Japan) using image software (Flouview 1000, Olympus, Tokyo, Japan).
The data is presented as the representative original recordings and graphs of the mean ± SEM. For statistical analysis, ANOVA followed by a post hoc t-test was applied (Figs. 1C, 2C, D). Unpaired t-test was applied to the corresponding data between control and test groups in Figs. 1B and 2B, and a P value < 0.05 was considered statistically significant.
All study protocols were in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and also conformed to Seoul National University College of Medicine guidelines for the care and use of animals. The animal protocol for experiments was reviewed and approved by the IACUC committee of the Seoul National University (SNU-090925-2).
An original trace of the Ussing chamber experiments with an ICR mouse is shown in Fig. 1A, in which the Vte and ΔVte are directly indicated. After the equilibrium period in NT solution, a stable level of Vte was confirmed. The initial spontaneous Vte was reduced by the luminal application of amiloride (10 µM), which blocks the epithelial Na+ channels (ENaC). In the presence of amiloride, cAMP-dependent Cl- secretion was induced via application of forskolin (2 µM) and isobutylmethyl xanthine (IBMX, 100 µM) to the basolateral side. The addition of forskolin/IBMX induced a negative shift in Vte and increased the lumen negative Isc. It is well known that luminal Cl- channels (CFTR) and basolateral K+ channels (KCNQ1) are activated by cAMP-dependent signalling pathways (17, 18). Consistent with this model, the application of the KCNQ1 channel blocker (100 µM of chromanol 293B) to the basolateral side suppressed the cAMP-induced Isc. Then, ATP (50 µM) was applied to the luminal side of the tracheal epithelium, which is known to induce a transient increase in cytosolic Ca2+ concentration ([Ca2+]c) via phospholipase C (PLC)/inositol-trisphosphate (InsP3)-coupled signalling pathways inducing the release of stored Ca2+ from the endoplasmic reticulum (ER) (19). This luminal ATP-induced increase in [Ca2+]c (Δ[Ca2+]c) is known to induce Cl- secretion via Ca2+-activated Cl- channels (ClCa) in the luminal membrane. Consistently, transient increases in Vte and Isc were observed in response to the luminal ATP application (19, 20). The Isc values measured in each phase of the above protocol showed a decreasing tendency in the mice exposed to strong hyperoxic conditions (PO2, 98%) for 36 hr (Fig. 1B).
The average values of Isc change (ΔIsc) induced by the above pharmacological treatments are summarized in Fig. 1C. In mice that were in hyperoxia conditions (80%, 90%, and 98% of PO2 for 36 hr), the peak amplitude of ΔIsc induced by forskolin/IBMX (ΔIsc,forsk) decreased at the highest PO2 condition compared to the responses of the mice exposed to 80% PO2 or to the normoxic condition (Fig. 1C). The peak increase in Isc due to the luminal ATP (ΔIsc,ATP) of 98% PO2 was smaller than that of 80% PO2 and not significantly different from that of the control (Fig. 1D). In contrast, the amiloride-sensitive Isc (ΔIsc,amil) was not affected by chronic hyperoxia (Fig. 1E).
Next, we investigated the Isc responses of airway epithelia obtained from wild-type C57BL/6 mice (WT), gulo(-/-) mice with vitamin C-supplementation for three weeks (K/O+Vit-C), and gulo(-/-) mice reared with no vitamin C-supplementation for 5-7 days (K/O-1wk), 14-15 days (K/O-2wk), 20-22 days (K/O-3wk), and 27-29 days (K/O-4wk). In Fig. 2A, the representative traces of Vte and ΔVte are shown for WT and K/O-3wk mice. Also, the averaged Isc values measured in each phase of the above protocol (control, amiloride, forskolin/IBMX, 293B, and ATP) are summarized for the WT and K/O-3wk mice (Fig. 2B). In general, the responses of airway epithelial Isc to the above pharmacological agents were smaller in WT mice than those in the ICR mice described above (Fig. 1B). Nevertheless, it was notable that the amplitudes of Isc were markedly suppressed in K/O-3wk mice (Fig. 2B).
The amplitudes of ΔIsc,forsk were compared between the groups of different duration of vitamin C deprivation. Overall, ΔIsc,forsk showed a decreasing tendency beginning with K/O-2wk mice, then became significant in K/O-3wk mice, and was completely abolished in K/O-4wk mice (Fig. 2C). ΔIsc,ATP was also decreased in the K/O-3wk and K/O-4wk mice (Fig. 2D). In contrast, ΔIsc,amil was abolished only in the K/O-4wk group (Fig. 1E). We also noted that the tissue resistance (Rte) showed a decreasing tendency in K/O-3wk mice and was significantly decreased in K/O-4wk mice (Fig. 2F).
The above results suggest that the Cl- secretory function of the mouse airway epithelium is susceptible to ambient levels of oxidative stress when the endogenous antioxidant is deficient. Because the cAMP-activated CFTR is the major pathway of Cl- secretion (11, 13), we compared the expression of CFTR in airway epithelia between WT and gulo(-/-) mice. Strong expression of CFTR in the luminal membrane was commonly observed in the control and K/O+Vit-C mice. However, consistent with the decrease in ΔIsc,forsk according to the extent of vitamin C deficiency, the expression of CFTR was decreased in K/O-2wk mice and was nearly absent in K/O-3wk and K/O-4wk mice (Fig. 3).
As seen from the inhibition of ΔIsc,forsk by 293B, KCNQ1 activity is also critical to the maintenance of Cl- secretion through CFTR (18). Therefore, we investigated whether KCNQ1 expression is altered in gulo(-/-) mice. However, the expression of KCNQ1 was persistently observed in K/O-3wk and K/O-4wk mice (Fig. 4). While not rigorously analyzed here, morphological changes were also observed in the airway epithelia of gulo (-/-) mice; the typical ciliated columnar epithelium became cuboid or flattened in response to increased duration of vitamin C deficiency (Figs. 3, 4, K/O-3wk and K/O-4wk).
In the present study, we found that chronic vitamin C deficiency suppressed Cl- secretion and downregulated CFTR expression in mouse airway epithelium. The cAMP-dependent Cl- secretion (ΔIsc,forsk) as well as the luminal ATP-induced Cl- secretion (ΔIsc,ATP) was decreased in gulo (-/-) mice. Since the CFTR was fully stimulated by forskolin/IBMX upon ATP application in the present experimental procedure, ΔIsc,ATP was believed to be due to Cl- secretion via CFTR as well as Ca2+-activated Cl- channels (19). In this respect, the decreased ΔIsc,ATP might also reflect the downregulation of CFTR. However, we could not conclude that the ion channels other than CFTR were actually affected by hyperoxia or vitamin C deficiency in mice. The inhibitory tendency of chronic hyperoxia on ΔIsc,Fsk also suggested that an imbalance between oxidizing influence and antioxidant capacity could impair the cAMP-dependent Cl- secretion mechanisms (Fig. 1C).
While CFTR-mediated secretion is more susceptible to vitamin C deficiency than it is to other parameters, the sustained deprivation of vitamin C generally suppresses the transepithelial transport functions. This functional impairment seems to precede the morphological changes in the epithelia of gulo(-/-) mice. Notably, the parameter reflecting the integrity of tissue (Rte) is severely lowered in K/O-4wk mice, indicating that it may be one of the scorbutic symptoms. It is well known that chronic deficiency of vitamin C weakens connective tissue due to impaired collagen synthesis (16). In addition to the decreased Rte, the histological findings of the K/O-3 and -4wk (flattened airway epithelium) mice indicate that a transformation from a ciliated/columnar epithelium occurs during the sustained loss of vitamin C in vivo.
The human respiratory tract is constantly exposed to transient instances of oxidative stress resulting from the inhalation of a variety of foreign materials including atmospheric pollutants and microorganisms. Furthermore, the production of ROS during episodes of infection and inflammation has been implicated in the pathogenesis of a number of pulmonary diseases such as asthma, adult respiratory distress syndrome, chromic obstructive pulmonary disease, and cystic fibrosis (21-24). Since inflammation and oxidative stress are closely related, inflammation also plays a role in the development of chronic lung disease (21, 22). Clinically, oxygen therapy is frequently applied to treat systemic hypoxemia. In this process, harmful effects of hyperoxia are often reported. The risk of hyperoxic stress and its sequel (e.g., bronchopulmonary dysplasia) are more prominently observed in neonates (2, 25).
The concomitant suppressions of ΔIsc,forsk and CFTR expression in vitamin C-deprived strongly suggest that chronic oxidative stress negatively regulates CFTR protein expression. Recently, it has been reported that oxidative stress induced by the pharmacological agent tert-butylhydroquinone (BHQ) suppresses CFTR expression in T84, a colonic epithelial cell line (6). The same group also reported that the functional expression of CFTR was suppressed in the human nasal mucosa of cigarette smokers (5). Based on the results of these previous studies, it is highly likely that the suppressions of ΔIsc,Fsk and CFTR in gulo(-/-) mice reflect a transcriptional regulation of CFTR genes under chronic oxidative stress (26, 27). Interestingly, CF-like symptoms such as thickened airway secretions are often seen in chronic inflammatory airway diseases that are not associated with mutations in the CFTR gene, and there is emerging evidence that posttranslational damage to CFTR by reactive oxygen and nitrogen species decreases CFTR function (27).
Antioxidants such as vitamin C may have beneficial effects via restoration of the balanced levels of ROS in tissues, as has been suggested in a study of hepatic ischemic-reperfusion injury model (28). Also, the lack of vitamin C in the ASL of asthmatics has been reported (9). However, careful interpretation is still required for the role of dietary supplementation of vitamin C in human, since there are controversies in the relation between plasma levels of vitamin C and cancer incidence (29). Furthermore, because we have not directly measured the oxidative stress in this study, the downregulation of CFTR by vitamin C deprivation might owe to the lack of vitamin C per se in addition to the putative oxidative stress.
In summary, this study confirms the inhibitory effects of vitamin C deprivation on the Cl- secretion function in murine airway epithelium in vivo. Among the ion channels associated with epithelial Cl- secretion, CFTR appears more to be vulnerable to oxidative stress than are other types of channels.
Figures and Tables
Fig. 1
Ussing chamber experiments in tracheal epithelia obtained from control and hyperoxia-exposed mice. (A) Original recordings of the transepithelial voltage (Vte). The upper border of the trace is Vte, the downward deflection (ΔVte) is the response to current injections from which the transepithelial resistance (Rte) and the equivalent short-circuit current (Isc) were calculated. The bars below indicate the luminal (lu) or basolateral (bl) application of amiloride (10 µM), forskolin (2 µM)/IBMX (100 µM), chromanol 293B (293B 10 µM) or ATP (50 µM). (B) Summary of Isc measured in the initial control and during each phase of drug application as demonstrated above. Open and closed bar graphs indicate results from normoxia (21% PO2) and hyperoxia (98% PO2, 36 hr)-exposed mice, respectively. (C-E) Summaries of the changes in Isc (ΔIsc) caused by forskolin/IBMX (C), amiloride (D), and ATP (E), as demonstrated in the above trace. Data from the groups of mice exposed to different levels of oxygen tension (21%, 80%, 90%, and 98% PO2) are compared. Numbers of tested tissues are directly indicated in the figure. The asterisks indicate statistical significance (P < 0.05, paired t-test).
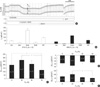
Fig. 2
Ussing chamber experiments in tracheal epithelia obtained from control and vitamin C-deficient mice. (A) Original recordings of the transepithelial voltages (Vte) in control (WT [C57BL/6], upper trace) and gulo(-/-) mice with no vitamin C supplementation for three weeks (K/O-3wk, lower trace). The bars below indicate the luminal (lu) or basolateral (bl) application of amiloride (10 µM), forskolin (2 µM)/IBMX (100 µM), chromanol 293B (293B 10 µM) or ATP (50 µM). (B) Summary of the Isc measured in the initial controls and during each phase of drug application, as demonstrated above. Open and closed bar graphs indicate results from WT (C57BL/6) and K/O-3wk, respectively. (C-E) Summaries of the changes in Isc (ΔIsc) caused by forskolin/IBMX (C), amiloride (D), and ATP (E), as demonstrated in the above trace. Data from the groups of WT and gulo(-/-) mice with or without vitamin C supplementation. The vitamin C deprivation period was varied from one to four weeks. Numbers of tested tissues are directly indicated in the figure. The asterisks indicate statistical significance (P < 0.05, paired t-test). (F) Summaries of tissue resistance (Rte) measured in each group.
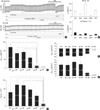
Fig. 3
Decreased expression of CFTR in the tracheal epithelia from vitamin C-deprived mice. Using confocal microscopy, the expression of CFTR (red) was examined with immunostaining in the tracheal epithelia of wild type (A), vitamin C-supplemented gulo(-/-) (B), and vitamin C-deprived gulo(-/-) mice (C-F). Nuclei were counterstained with DAPI (blue). The expressions of CFTR in luminal membranes decreased in K/O-2wk (D) and almost disappeared in K/O-3wk mice (E). Note that the columnar shape of the epithelium changes to cube-like or flattened in K/O-4wk (F). Scale bar, 50 µm.
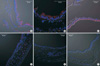
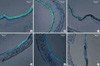
Fig. 4
Persistent expression of KCNQ1 in the tracheal epithelia from vitamin C-deprived mice. Immunofluorescence microcopy was used to compare the expressions of KCNQ1 (green) in WT (A) and gulo(-/-) mice in which vitamin C was either supplemented (B) or not for 1, 2, 3, and 4 weeks (C-F). Nuclei were counterstained with DAPI (blue). Scale bar, 50 µm.
AUTHOR SUMMARY
Suppression of CFTR-mediated Cl- Secretion of Airway Epithelium in Vitamin C-deficient Mice
Yeryung Kim, Hyemin Kim, Hae-Young Yoo, Jae Seung Kang, Sung Joon Kim, Jin Kyoung Kim, and Hyun Sung Cho
During surgery or post-operative care, prolonged high oxygen (hyperoxia) supply might damage respiratory system, which is mediated by oxidative stress. Airway epithelium is covered by a thin fluid that is maintained by cooperative actions of membrane ion channels such as cystic fibrosis conductance regulator (CFTR) and K+ channel (KCNQ1). The CFTR activity of mouse airway epithelia was attenuated when exposed to 98% oxygen for a prolonged period (> 36 hr). Vitamin C, an antioxidant intrinsically produced in mice, might play a protective role. In fact, the CFTR activity in the vitamin C-depleted scurvy mice (gulo(-/-) mice) was decreased in normal ambient air. An immunofluorescence study confirmed the decreased expression of CFTR in gulo(-/-) mice whereas KCNQ1 was preserved. The CFTR-depend! ent airway surface fluid is susceptible to oxidative stress, which might suggest the benefit of vitamin C supplementation.
References
1. Saugstad OD. Chronic lung disease: oxygen dogma revisited. Acta Paediatr. 2001. 90:113–115.
2. Sola A. Oxygen in neonatal anesthesia: friend or foe? Curr Opin Anaesthesiol. 2008. 21:332–339.
3. Brozmanova M, Plevkova J, Bartos V, Plank L, Javorka M, Tatar M. The interaction of dietary antioxidant vitamins and oxidative stress on cough reflex in guinea-pigs after long term oxygen therapy. J Physiol Pharmacol. 2006. 57:Suppl 4. 45–54.
4. Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. 2007. 4th ed. Oxford: Oxford University Press;1–29.
5. Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006. 173:1139–1144.
6. Cantin AM, Bilodeau G, Ouellet C, Liao J, Hanrahan JW. Oxidant stress suppresses CFTR expression. Am J Physiol Cell Physiol. 2006. 290:C262–C270.
7. Jeulin C, Guadagnini R, Marano F. Oxidant stress stimulates Ca2+-activated chloride channels in the apical activated membrane of cultured nonciliated human nasal epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005. 289:L636–L646.
8. Mohsenin V. Effect of vitamin C on NO2-induced airway hyperresponsiveness in normal subjects. A randomized double-blind experiment. Am Rev Respir Dis. 1987. 136:1408–1411.
9. Kelly FJ, Mudway I, Blomberg A, Frew A, Sandström T. Altered lung antioxidant status in patients with mild asthma. Lancet. 1999. 354:482–483.
10. van der Vliet A, O'Neill CA, Cross CE, Koostra JM, Volz WG, Halliwell B, Louie S. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am J Physiol. 1999. 276:L289–L296.
11. Chambers LA, Rollins BM, Tarran R. Liquid movement across the surface epithelium of large airways. Respir Physiol Neurobiol. 2007. 159:256–270.
12. Fischer H, Schwarzer C, Illek B. Vitamin C controls the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci U S A. 2004. 101:3691–3696.
13. Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004. 23:146–158.
14. Cowley EA, Linsdell P. Oxidant stress stimulates anion secretion from the human airway epithelial cell line Calu-3: implications for cystic fibrosis lung disease. J Physiol. 2002. 543:201–209.
15. Schwarzer C, Fischer H, Kim EJ, Barber KJ, Mills AD, Kurth MJ, Gruenert DC, Suh JH, Machen TE, Illek B. Oxidative stress caused by pyocyanin impairs CFTR Cl(-) transport in human bronchial epithelial cells. Free Radic Biol Med. 2008. 45:1653–1662.
16. Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci USA. 2000. 97:841–846.
17. Cotton CU. Basolateral potassium channels and epithelial ion transport. Am J Respir Cell Mol Biol. 2000. 23:270–272.
18. Grahammer F, Warth R, Barhanin J, Bleich M, Hug MJ. The small conductance K+ channel, KCNQ1: expression, function, and subunit composition in murine trachea. J Biol Chem. 2001. 276:42268–42275.
19. Schreiber R, Kunzelmann K. Purinergic P2Y6 receptors Induce Ca2+ and CFTR dependent Cl- secretion in mouse trachea. Cell Physiol Biochem. 2005. 16:99–108.
20. Paradiso AM, Ribeiro CM, Boucher RC. Polarized signaling via purinoceptors in normal and cystic fibrosis airway epithelia. J Gen Physiol. 2001. 117:53–67.
21. Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J. 2000. 16:534–554.
22. van der Vliet A, Cross CE. Oxidants, nitrosants, and the lung. Am J Med. 2000. 109:398–421.
23. Dworski R. Oxidant stress in asthma. Thorax. 2000. 55:Suppl 2. S51–S53.
24. Lamb NJ, Gutteridge JM, Baker C, Evans TW, Quinlan GJ. Oxidative damage to proteins of bronchoalveolar lavage fluid in patients with acute respiratory distress syndrome: evidence for neutrophil-mediated hydroxylation, nitration, and chlorination. Crit Care Med. 1999. 27:1738–1744.
25. Powers WF, Clemens J. Risk factors for bronchopulmonary dysplasia. J Pediatr. 1992. 120:667–668.
26. Cantin AM, White TB, Cross CE, Forman HJ, Sokol RJ, Borowitz D. Antioxidants in cystic fibrosis. Conclusions from the CF antioxidant workshop, Bethesda, Maryland, November 11-12, 2003. Free Radic Biol Med. 2007. 42:15–31.
27. Bebok Z, Varga K, Hicks JK, Venglarik CJ, Kovacs T, Chen L, Hardiman KM, Collawn JF, Sorscher EJ, Matalon S. Reactive oxygen nitrogen species decrease cystic fibrosis transmembrane conductance regulator expression and cAMP-mediated Cl- secretion in airway epithelia. J Biol Chem. 2002. 277:43041–43049.
28. Rhee JE, Jung SE, Shin SD, Suh GJ, Noh DY, Youn YK, Oh SK, Choe KJ. The effects of antioxidants and nitric oxide modulators on hepatic ischemic-reperfusion injury in rats. J Korean Med Sci. 2002. 17:502–506.
29. Lee GJ, Chung HW, Lee KH, Ahn HS. Antioxidant vitamins and lipid peroxidation in patients with cervical intraepithelial neoplasia. J Korean Med Sci. 2005. 20:267–272.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download