Abstract
Tissue genotyping is more useful approach than using blood genomic DNA, which can reflect the effects of the somatic mutations in cancer. Although polymorphisms in glutathione S-transferase (GST) have been associated with the risk of bladder cancer (BC) development, few reports provide information about the prognosis of BC. We investigated glutathione S-transferase mu (GSTM1) and glutathione S-transferase theta (GSTT1) genotypes using genomic DNA from primary 165 BC tissue samples to assess the association with disease prognosis. DNA samples from tumor were analyzed by multiplex polymerase chain reaction (PCR). The results were compared with clinicopathological parameters. The prognostic significance of the GSTs was evaluated by Kaplan-Meier and multivariate Cox regression model. Kaplan-Meier estimates revealed significant differences in time to tumor recurrence according to the GSTM1 tissue genotype (P = 0.038) in non-muscle invasive bladder cancer (NMIBC). Multivariate Cox regression analysis also revealed that the tissue GSTM1 genotype (hazards ratio [HR]: 0.377, P = 0.031) was an independent predictor of bladder tumor recurrence in NMIBC. This identification of GSTM1 tissue genotype as a prognosticator for determining recurrence in NMIBC should prove highly useful in a clinical setting.
Bladder cancer (BC) involves a heterogeneous cell population, and numerous factors are likely to be involved in tumorigenesis (1). These factors result in uncontrolled growth of the cell population, decreased cell death, invasion and metastasis, and may influence the patient's prognosis. Identification of the aggressive features of the cancer in patients with BC is very important for adequate management of this disease (2). While several molecular markers for the development, recurrence and progression of BC have been identified (3, 4), the limited value of these established prognostic markers has emphasized the need for novel molecular indicators of BC outcomes.
Previous molecular epidemiologic studies have revealed that polymorphisms in glutathione S-transferase-mu (GSTM1) and glutathione S-transferase-theta (GSTT1) have been implicated as risk factors in BC (5, 6). Furthermore, a small number of reports have added valuable information towards our understanding of the clinicopathologic characteristics of BC (7, 8).
Several environmental factors, as well as the activities of detoxification enzymes, have been associated with tumor development in BC, and could also affect the clinical course of this disease. To date, there has been no published report describing the prognostic value of homozygous deletions of GSTM1 and GSTT1 in tumor tissues as markers of disease prognosis in primary BC patients with long-term follow-up. This study is the first and the only study that used tumor tissue to analyze the GSTM1 and GSTT1 genotypes to evaluate the prognosis of BC instead of blood genomic DNA. In the current study, we identified the GSTM1 tissue genotype as a prognosticator of recurrence in patients with primary non-muscle invasive bladder cancer (NMIBC).
We used primary BC tissue samples taken all from the initial primary tumor resection (no prior intravesical therapy or systemic adjuvant chemotherapy) from 165 consecutive cases of patients with histologically-verified transitional cell carcinoma, which were obtained from Chungbuk National University Hospital between 1995 and 2007. To reduce confounding factors that may affect the analyses, any patients diagnosed with concomitant carcinoma in situ (CIS) lesion or CIS lesion alone were excluded. All tumors were macro-dissected, typically within 15 min of surgical resection. Each BC specimen was confirmed by pathological analysis of a part of the tissue sample in fresh-frozen sections from cystectomy and transurethral resection (TUR) specimens, and then frozen in liquid nitrogen and stored at -80℃ until use.
Tumors were staged according to the 2002 TNM classification (9) and the 1973 WHO grading system (10). In the case of NMIBC, TUR of the tumor was performed. A second TUR was performed 2-4 weeks after the initial resection when a BC specimen did not include proper muscle or when a high-grade was detected (11). Patients who had multiple tumors, large tumors (≥ 3 cm in diameter), or high grade NMIBC received one cycle of intravesical treatment (Bacillus Calmette-Guérin [BCG] or mitomycin-C) (11, 12). Response to treatment was assessed by cystoscopy and urinary cytology. Patients who were free of disease within 3 months of commencement of treatment were assessed every 3 months for the first 2 yr and every 6 months thereafter (11, 12). We defined recurrence as the relapse of primary NMIBC with a lower or equivalent pathologic stage, and progression as disease with a higher TNM stage when relapsed. In cases of muscle-invasive bladder cancer (MIBC), radical cystectomy and complete pelvic lymph node dissection were performed. Patients with pT3, pT4, or node-positive disease, based on the analysis of radical cystectomy specimens, received at least four cycles of cisplatin-based chemotherapy. Either clinically metastatic disease, or non-cystectomy cases were excluded in this study. Each patient has been followed and managed according to the standard recommendation (13). Among MIBC cases, we defined progression as local regional recurrence or metastasis after radical cystectomy and adequate adjuvant chemotherapy.
Genomic DNA was isolated from frozen tumor tissue specimens using the Wizard Genomic DNA Purification System Kit (Promega, Maddison, WI, USA) in accordance with the manufacturer's instructions. A multiplex PCR method was used to detect the presence or absence of the GSTM1 and GSTT1 polymorphisms in genomic DNA samples, as described previously (14). The primers used in the PCR were: 1) GSTM1 (219 bp) sense (5'-GAA CTC CCT GAA AAG CTA AAG C-3') and anti-sense (5'-GTT GGG CTC AAA TAT ACG GTG G-3'); 2) GSTT1 (372 bp) sense (5'-TTA GCT GAC CTC GTA GCC AT-3') and anti-sense (5'-GAA GTC CTT GGC CTT CAG AA-3'); and 3) β-globin (268 bp) sense (5'-GAA GAG CCA AGG ACA GGT AC-3') and anti-sense (5'-CAA CTT CAT CCA CGT TCA CC-3'). DNA (200 ng) was amplified in a total volume of 20 µL, containing 10 pM of each primer, 0.5 unit of Taq polymerase, 2.5 mM dNTP, and 10 × PCR buffer. Following an initial denaturation step at 94℃ for 5 min, 40 cycles of amplification were performed at: 1) 94℃ for 60 sec; 2) 63℃ for 60 sec; and 3) 72℃ for 60 sec. A final extension step was then performed at 72℃ for 10 min. Confirmation of PCR reactions was performed by electrophoresis of the amplified products on a 2% agarose gel. Positive and negative control samples were analyzed in each experiment. GSTM1 and GSTT1 genotypes were only scored in the presence of the internal reference gene (β-globin) product.
The chi-square test and Fisher's exact test were used to analyze the frequencies of the genotypes according to clinicopathological parameters. The Kaplan-Meier method was used to estimate time to recurrence and progression, and differences were assessed using log-rank statistics. The prognostic value of the GSTM1 and GSTT1 genotypes for recurrence and progression of BC was analyzed with multivariate Cox proportional hazard regression models. Statistical analysis was performed using SPSS 12.0 software (SPSS Inc., Chicago, IL, USA), and a P value of < 0.05 was considered statistically significant.
Table 1 lists the baseline characteristics of the 165 patients enrolled in this study. The median follow-up period of the primary BC patients was 57.3 months (range, 1.0-161.4). Total 20 of 102 patients (19.6%) received the repeat TUR for the adequate tumor staging. Intravesical therapy was performed in 50 patients (49.0%) after primary TUR including 39 patients treated with BCG, and 11 with mitomycin-C. In our study, patients who completed 6 times of intravesical therapy were counted in number. Fig. 1 shows 2% agarose gel demonstrating multiplex PCR genotyping of genomic DNA samples for the detection of GSTM1 and GSTT1 gene deletion. The frequencies of the GSTM1-null and GSTT1-null types were 69.1% and 47.9%, respectively.
Table 2 summarizes the correlations between genotype and clinicopathological parameters in patients with NMIBC. The GSTM1-null genotype was observed in 30 of 36 NMIBCs (83.3%) with recurrence and in 41 of 66 (62.1%) without recurrence, which represented a statistically significant difference (P = 0.026). No significant correlations were observed between GSTM1 genotype and the other clinicopathological parameters such as tumor stage, grade, tumor size, multiplicity and progression. The GSTT1-positive genotype was more frequently detected in large-sized (≥ 3 cm) than in small-sized NMIBC (< 3 cm) (28 of 46 or 60.9% vs 21 of 56 or 37.5%, P = 0.019). The other parameters were not significantly associated with GSTT1 genotype. Associations between the genotype of GSTM1 or GSTT1 and clinicopathological features of MIBC were not found (Table 3).
Kaplan-Meier estimates revealed a significant difference in time to tumor recurrence according to the GSTM1 genotype (log-rank test, P = 0.038, Fig. 2). However, recurrence-free survival was not related to the GSTT1 genotype. Multivariate Cox regression analysis revealed that the GSTM1 genotype was an independent predictor of bladder tumor recurrence (hazard ratio, 0.377; 95% confidence interval, 0.156-0.914; P = 0.031, Table 4).
The activities of specific enzymes can change with genotype. GSTM1 and GSTT1 are involved in cellular metabolism and detoxification of carcinogenic products, and these detoxification related-enzymes are associated with tumorigenesis of BC (5, 6, 15, 16). Specific enzymes that are known to be important in carcinogenesis can also play a critical role in disease recurrence and progression after initial treatment.
In this study, we investigated the GSTM1 and GSTT1 genotypes in human primary BC tissues. The results demonstrated that the GSTM1 tissue genotype was a strong indicator for predicting recurrence in patients with primary NMIBC. However, the GSTM1 genotype was not associated with tumor progression. Previous blood genotype studies revealed that individuals with the GSTM1-null genotype were at great risk of developing BC (5, 6, 16). These results imply that the GSTM1-null genotype may contribute to the initiation of tumorigenesis in BC simply by promoting cell proliferation, rather than by the generation of certain aspects of the aggressive phenotype such as progression and invasion. There have been several studies aimed at identifying the biologic potential of bladder tumors, which may help to better predict the clinical outcome of BC, including recurrence and progression (17-20). Since the molecular genetic aspects are different between recurrence and progression in NMIBC (21-23), it seems necessary to discriminate these two pathogeneses. Grossman et al. reported that p53 and retinoblastoma (RB) expression in T1 NMIBC can be used to predict progression only, and not recurrence without progression (19). Kim et al. (20) suggested that RUNX3 methylation was correlated with disease progression but did not detect a statistically significant association between RUNX3 methylation and recurrence in NMIBC. A small number of reports have described tumor markers related to recurrence without progression in NMIBC (17, 18), however, cell cycle markers were reported to provide no added prognostic information on tumor recurrence in NMIBC (17). Van Rhijn et al. (18) suggested that FGFR3 mutation was a strong indicator for predicting recurrence in NMIBC. However, the design of that study carried limitations in terms of a relatively small sample size and in including both primary and recurrence cases. In the present study, we used only 165 primary BC tissues with long-term follow-up. In our data, the conventional prognostic factors such as multiplicity, tumor size, stage and grade did not related to the recurrence in NMIBC on multivariate analysis. Although we do not know the exact reason, one possibility is that the patients at high risk of recurrence or progression underwent second TUR or intravesical therapy, and these adjuvant treatments might act as the confounding effects against the conventional prognostic factors.
Most of the single nucleotide polymorphism (SNP) studies of GSTM1 and GSTT1 have been case control studies, or cross-sectional studies based on epidemiology. There are several distinctive aspects of the current study compared with previous SNP studies. First, we used genomic DNA from 165 primary BC tissue samples for the analysis. To date, there has been no study using tumor tissues to identify the genotype of GSTM1 and GSTT1. DNA from tumor tissues represents the effects of the somatic mutations, alterations occurring in tumor DNA could affect the gene expression. We have the expression analysis data for GSTM1 and GSTT1 using tumor DNA. The mRNA expression of GSTM1 and GSTT1 was higher in BC tissues with positive genotypes than in those with null genotypes (24). It also revealed that tumor genotype might affect the expression of the specific gene. In these regards, we suggest that using genomic DNA from tumor tissues to the genotypes of GSTM1 and GSTT1 is a more important and useful approach than using genomic DNA from blood which could also be affected by changes in the patient's health status originating from other diseases unrelated to the cancer that investigators are attempting to analyze. Second, we performed definitive subgroup analysis and survival analyses such as Kaplan-Meier and Cox regression analysis with long-term follow-up. Only a small number of studies have reported GSTM1 and GSTT1 genotype according to the stage and grade of the BC (7, 8, 25), and the results were inconsistent among the different ethnic groups. We analyzed all the factors affecting BC prognosis, including stage, grade, multiplicity and size, in conjunction with GSTM1 and GSTT1 tissue genotypes. We found that the GSTT1-positive genotype was more frequently detected in large-sized (≥ 3 cm) than in small-sized NMIBC (< 3 cm). This finding supports our previous data showing that a GSTT1-null genotype is not a risk factor, but a protective factor, for BC (15). The GSTT1-positive genotype may reflect the aggressiveness of NMIBC, but further investigation to confirm this proposal will be needed.
Frequent recurrence and progression are devastating events for both urologists and BC patients. The high incidence of recurrence results in considerable costs that make NMIBC one of the most expensive diseases to treat (26). Progression from NMIBC to MIBC or metastasis is not unusual and is often life-threatening to the patient. Efforts towards preventing these events are ongoing. The traditional methods include a second TUR, intravesical drug instillation treatment, and early cystectomy (27, 28). Some reports have revealed that early cystectomy results in a superior 5-yr survival rate in comparison with bladder-sparing surgery (2). Cystectomy may lead to severe complications or morbidity (29), and there has been much controversy as to whether cystectomy represents over-treatment (30). If patients with NMIBC are likely to suffer from recurrence of the disease, but will never experience progression, the need for early cystectomy is diminished. Therefore, the usefulness of GSTM1 tissue genotype as a recurrence prognosticator demonstrated in the current study must be emphasized to urologists. However, introducing this prognostic test for NMIBC into routine clinical practice requires further external validation in a prospective manner using a large number of samples.
In conclusion, we showed that the GSTM1 tissue genotype has a predictive value for determining recurrence in NMIBC. It is suggested that the GSTM1 tissue genotype may play an important role in the prognosis of NMIBC in the clinical setting in the future.
Figures and Tables
Fig. 1
Electrophoretic findings of GSTM1 and GSTT1, Lane M: molecular size marker (100 bp DNA ladder); Lane 1: GSTM1-null/GSTT1-null type; Lane 2: GSTM1-null/GSTT1-positive type; Lane 3: GSTM1-positive/GSTT1-null type; Lane 4: GSTM1-positive/GSTT1-positive type.
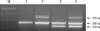
Fig. 2
Kaplan-Meier estimate curves predict the probability of recurrence according to the GSTM1 genotype in patients with non-muscle invasive bladder cancer.

Table 2
Relationship between GSTM1 and GSTT1 genotypes, and clinicopathological parameters in non-muscle-invasive bladder cancer
AUTHOR SUMMARY
GSTM1 Tissue Genotype as a Recurrence Predictor in Non-muscle Invasive Bladder Cancer
Yun-Sok Ha, Chunri Yan, Pildu Jeong, Won Tae Kim, Seok-Joong Yun, Isaac Yi Kim, Sung-Kwon Moon, and Wun-Jae Kim
Tissue genotyping is more useful approach than using blood genomic DNA, which can reflect the effects of the somatic mutations in cancer. We investigated the genotype of GSTM1 and GSTT1 in bladder cancer (BC) using genomic DNA from 165 BC tissue samples, to assess the association with disease prognosis in BC. Kaplan-Meier estimates revealed significant differences in time to tumor recurrence according to the GSTM1 tissue genotype (P = 0.038) in non-muscle invasive bladder cancer (NMIBC). Multivariate Cox regression analysis also revealed that the tissue GSTM1 genotype (hazards ratio [HR]: 0.377, P = 0.031) was an independent predictor of bladder tumor recurrence in NMIBC. This identification of GSTM1 tissue genotype as a prognosticator for determining recurrence in NMIBC might prove highly useful in a clinical setting.
References
1. Hirao Y, Kim WJ, Fujimoto K. Environmental factors promoting bladder cancer. Curr Opin Urol. 2009. 19:494–499.
2. Borden LS Jr, Clark PE, Hall MC. Bladder cancer. Curr Opin Oncol. 2003. 15:227–233.
3. Kim TH, Jo SW, Lee YS, Kim YJ, Lee SC, Kim WJ, Yun SJ. Forkhead box O-class 1 and forkhead box G1 as prognostic markers for bladder cancer. J Korean Med Sci. 2009. 24:468–473.
4. Ha YS, Kim MJ, Yoon HY, Kang HW, Kim YJ, Yun SJ, Lee SC, Kim WJ. mRNA Expression of S100A8 as a prognostic marker for progression of non-muscle-invasive bladder cancer. Korean J Urol. 2010. 51:15–20.
5. Kim WJ, Lee HL, Lee SC, Kim YT, Kim H. Polymorphisms of N-acetyltransferase 2, glutathione S-transferase mu and theta genes as risk factors of bladder cancer in relation to asthma and tuberculosis. J Urol. 2000. 164:209–213.
6. Brockmöller J, Cascorbi I, Kerb R, Roots I. Combined analysis of inherited polymorphisms in arylamine N-acetyltransferase 2, glutathione S-transferases M1 and T1, microsomal epoxide hydrolase, and cytochrome P450 enzymes as modulators of bladder cancer risk. Cancer Res. 1996. 56:3915–3925.
7. Song DK, Xing DL, Zhang LR, Li ZX, Liu J, Qiao BP. Association of NAT2, GSTM1, GSTT1, CYP2A6, and CYP2A13 gene polymorphisms with susceptibility and clinicopathologic characteristics of bladder cancer in Central China. Cancer Detect Prev. 2009. 32:416–423.
8. Guey LT, García-Closas M, Murta-Nascimento C, Lloreta J, Palencia L, Kogevinas M, Rothman N, Vellalta G, Calle ML, Marenne G, Tardón A, Carrato A, García-Closas R, Serra C, Silverman DT, Chanock S, Real FX, Malats N. EPICURO/Spanish Bladder Cancer Study investigators. Genetic susceptibility to distinct bladder cancer subphenotypes. Eur Urol. 2010. 57:283–292.
9. Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg. 2002. 87:13–15.
10. Mostofi FK, Sobin LH, Torloni H. Histological typing of urinary bladder tumors. International Histologic Classification of Tumors. 1973. Geneva: World Health Organzation.
11. Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou-Redorta J. European Association of Urology (EAU). EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2008. 54:303–314.
12. Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, Wolf JS Jr, Schellhammer PF. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007. 178:2314–2330.
13. Stenzl A, Cowan NC, De Santis M, Jakse G, Kuczyk MA, Merseburger AS, Ribal MJ, Sherif A, Witjes JA. The updated EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2009. 55:815–825.
14. Chen H, Sandler DP, Taylor JA, Shore DL, Liu E, Bloomfield CD, Bell DA. Increased risk for myelodysplastic syndromes in individuals with glutathione transferase theta 1 (GSTT1) gene defect. Lancet. 1996. 347:295–297.
15. Kim WJ, Kim H, Kim CH, Lee MS, Oh BR, Lee HM, Katoh T. GSTT1-null genotype is a protective factor against bladder cancer. Urology. 2002. 60:913–918.
16. Bell DA, Taylor JA, Paulson DF, Robertson CN, Mohler JL, Lucier GW. Genetic risk and carcinogen exposure: a common inherited defect of the carcinogen-metabolism gene glutathione S-transferase M1 (GSTM1) that increases susceptibility to bladder cancer. J Natl Cancer Inst. 1993. 85:1159–1164.
17. Pfister C, Moore L, Allard P, Larue H, Lacombe L, Têtu B, Meyer F, Fradet Y. Predictive value of cell cycle markers p53, MDM2, p21, and Ki-67 in superficial bladder tumor recurrence. Clin Cancer Res. 1999. 5:4079–4084.
18. van Rhijn BW, Lurkin I, Radvanyi F, Kirkels WJ, van der Kwast TH, Zwarthoff EC. The fibroblast growth factor receptor 3 (FGFR3) mutation is a strong indicator of superficial bladder cancer with low recurrence rate. Cancer Res. 2001. 61:1265–1268.
19. Grossman HB, Liebert M, Antelo M, Dinney CP, Hu SX, Palmer JL, Benedict WF. p53 and RB expression predict progression in T1 bladder cancer. Clin Cancer Res. 1998. 4:829–834.
20. Kim EJ, Kim YJ, Jeong P, Ha YS, Bae SC, Kim WJ. Methylation of the RUNX3 promoter as a potential prognostic marker for bladder tumor. J Urol. 2008. 180:1141–1145.
21. Kim WJ, Kim EJ, Kim SK, Kim YJ, Ha YS, Jeong P, Kim MJ, Yun SJ, Lee KM, Moon SK, Lee SC, Cha EJ, Bae SC. Predictive value of progression-related gene classifier in primary non-muscle invasive bladder cancer. Mol Cancer. 2010. 9:3.
22. Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005. 5:713–725.
23. Kim YJ, Ha YS, Kim SK, Yoon HY, Lym MS, Kim MJ, Moon SK, Choi YH, Kim WJ. Gene signatures for the prediction of response to Bacillus Calmette-Guerin immunotherapy in primary pT1 bladder cancers. Clin Cancer Res. 2010. 16:2131–2137.
24. Ha YS, Yan C, Park C, Yun SJ, Moon SK, Choi YH, Kim WJ. GSTT1: A marker of the aggressiveness of bladder cancer. Urol Int. 2010. doi: 10.1159/000321689.
25. Srivastava DS, Mishra DK, Mandhani A, Mittal B, Kumar A, Mittal RD. Association of genetic polymorphism of glutathione S-transferase M1, T1, P1 and susceptibility to bladder cancer. Eur Urol. 2005. 48:339–344.
26. Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003. 21:1315–1330.
27. Divrik RT, Yildirim U, Zorlu F, Ozen H. The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycin: a prospective, randomized clinical trial. J Urol. 2006. 175:1641–1644.
28. Thalmann GN, Markwalder R, Shahin O, Burkhard FC, Hochreiter WW, Studer UE. Primary T1G3 bladder cancer: organ preserving approach or immediate cystectomy? J Urol. 2004. 172:70–75.
29. Ramani VA, Bromage SJ, Clarke NW. A contemporary standard for morbidity and outcome after radical cystectomy. BJU Int. 2009. 104:628–632.
30. Shariat SF, Palapattu GS, Karakiewicz PI, Rogers CG, Vazina A, Bastian PJ, Schoenberg MP, Lerner SP, Sagalowsky AI, Lotan Y. Concomitant carcinoma in situ is a feature of aggressive disease in patients with organconfined TCC at radical cystectomy. Eur Urol. 2007. 51:152–160.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download