Abstract
HMG-CoA reductase inhibitors (statins) are widely used to treat hypercholesterolemia. Among the adverse effects associated with these drugs are statin-associated myopathies, ranging from asymptomatic elevation of serum creatine kinase to fatal rhabdomyolysis. Fluvastatin-induced fatal rhabdomyolysis has not been previously reported. We describe here a patient with liver cirrhosis who experienced fluvastatin-induced fatal rhabdomyolysis. This patient had been treated with simvastatin (20 mg/day) for coronary artery disease and was switched to fluvastatin (20 mg/day) 10 days before admission. He was also taking aspirin, betaxolol, candesartan, lactulose, and entecavir. Rhabdomyolysis was complicated and continued to progress. He was treated with massive hydration, urine alkalization, intravenous furosemide, and continuous renal replacement therapy for acute renal failure, but eventually died due to rhabdomyolysis complicated by hepatic failure. In conclusion, fluvastatin should be used with caution in patients with liver cirrhosis, especially with other medications metabolized with CYP2C9.
Statins are used to lower serum cholesterol concentrations in the primary and secondary prevention of cardiovascular disease (1). In randomized trials, the use of statins has demonstrated 30% reductions in atherosclerotic end points without serious morbidity. The pleiotropic effects of statins, indirectly related to cholesterol-lowering activities (2), have also been assessed, making these drugs more widely accepted in various clinical settings.
Fluvastatin, available as both fluvastatin sodium and fluvastatin extended-release, is the first synthetic 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Because fluvastatin is well tolerated and lacks clinically significant drug-drug interactions, it is regarded as one of the safest statins, especially in patients receiving multiple medications (3). Even patients with renal disease, renal transplantation recipients and patients who have undergone PCI can be safely treated with fluvastatin (4).
The most significant adverse effect associated with statins is muscle toxicity. This includes a broad clinical spectrum of disorders, from mild and non-specific muscle aches or weakness, through asymptomatic and symptomatic elevation of serum creatine kinase levels, to life-threatening rhabdomyolysis. To date, few cases of fatal rhabdomyolysis associated with the use of statins have been reported; these include 19 patients taking lovastatin, 3 taking pravastatin, 14 taking simvastatin, 6 taking atorvastatin, and 31 taking cerivastatin, the latter which was withdrawn worldwide (5). There have been no reports to date of fatal rhabdomyolysis associated with the use of fluvastatin. We report a case of fluvastatin-induced fatal rhabdomyolysis in a patient with liver cirrhosis; this patient had been switched from simvastatin to fluvastatin 10 days earlier and died due to severe rhabdomyolysis complicated by hepatic failure.
A 56-yr-old man with liver cirrhosis caused by the hepatitis B virus visited our hospital on December 30, 2008 for evaluation of weakness in his lower legs of 1-week duration. He first experienced discomfort and myalgia in his lower legs, which worsened over time and made him unable to walk. He had undergone a percutaneous coronary intervention 10 yr previously at the local hospital and had taken simvastatin (20 mg/day) regularly since then. He was diagnosed with Child-Pugh C cirrhosis due to hepatitis B virus a year ago and followed-up at the same hospital with stable liver function in a compensated state. Ten days before visiting our hospital, he was switched from simvastatin to fluvastatin (20 mg/day) by his doctor, as the patient was concerned about his liver disease. In addition to fluvastatin (20 mg/day), his medications included aspirin (100 mg/day), betaxolol (10 mg/day), candesartan (16 mg/day), lactulose (30 mL/day), and entecavir (0.5 mg/day) without diuretics or fibrates. His medical history was significant for no alcohol use and 10 pack-years of smoking, but he had quit smoking 10 yr previously.
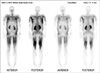
The patient's blood pressure was 122/76 mmHg, his temperature was 36.8℃, his pulse was 63 beats/min, weight was 98 kg, and his body mass index was 29.8 kg/m2. He had mild tenderness of the lower extremities but all other assessments were unremarkable. Laboratory findings included sodium 125 mM/L, potassium 5.1 mM/L, chloride 97 mM/L, phosphorus 3.1 mg/dL, CO2 24.4 mM/L, serum creatinine 1.0 mg/dL, blood urea nitrogen 21 mg/dL, glucose 176 mg/dL, calcium 8.3 mg/dL, phosphorus 3.1 mg/dL, aspartate transaminase 1,303 IU/L (normal < 40), alanine transaminase 354 IU/L (normal < 40), alkaline phosphatase 145 IU/L (normal 40 to 120), total bilirubin 4.6 mg/dL, direct bilirubin 1.4 mg/dL, protein 8.0 g/dL, albumin 2.7 g/dL, and uric acid 3.5 mg/dL. His hematocrit was 38.5%, platelet count was 80,000/µL, and white blood cell count was 11,100/µL, with 18.5% lymphocytes, 69.7% polymorphonuclear leukocytes, 10.% monocytes, 1.4% eosinophils, and 0.2% basophils. His prothrombin time (PT) was 1.78 INR (40.7%) and activated partial thromboplastin time (aPTT) was 36.5 seconds. His total serum creatine kinase (CK) was 36,804 IU/L (normal 50 to 250 IU/L) and his CK-MB was 157.0 ng/mL (normal < 5 ng/mL). Thyroid function test was within normal limits. Urinalysis revealed no red or white blood cells, positive dipsticks for protein, occult blood, bilirubin and urobilinogen, but negative for glucose. A 99mTc-diphosphonate scintigram bone scan showed increased uptake over all extremities and muscles of the thorax and abdomen (Fig. 1).
The patient was diagnosed with fluvastatin-induced rhabdomyolysis. Treatment with fluvastatin, betaxolol and candesartan was discontinued, and he received massive hydration and urine alkalization. He was treated with intravenous sodium bicarbonate (100 mEq in 1,000 mL of half-normal saline at a rate of up to 500 mL/h or 50 mEq/h) titrated to a urine pH > 6.5. He was administered a 20-80 mg dose of intravenous furosemide to maintain diuresis. The extent of rhabdomyolysis progressed despite supportive treatment. His CK concentration was increased to 166,160 IU/L, and renal failure occurred. He underwent continuous renal replacement therapy on day 7, but his renal function did not recover and hepatic function worsened, with PT prolonged to 3.8 INR (18%) and total bilirubin increased to 24.5 mg/dL (Fig. 2). He died due to rhabdomyolysis complicated by hepatic failure on day 15.
Fluvastatin is one of the statins shown to reduce the incidence of vascular events and slow the angiographically demonstrated progression of atherosclerotic disease (4). Since its approval in 1993, fluvastatin has been administered for more than 21 million patient-years worldwide. Among the statins, fluvastatin has been associated with the fewest cases of rhabdomyolysis. Although there was a report of fatal rhabdomyolysis induced by fluvastatin-gemfibrozil combination therapy (6), to our knowledge, fluvastatin alone has not been reported to induce fatal rhabdomyolysis.
Our patient had both chronic liver disease and established coronary artery disease, making it difficult to choose the most appropriate type and dose of statin, or even whether to treat with statin. The benefits of statins in lowering cholesterol and preventing heart disease outweigh the potential risks of hepatotoxicity, even in patients with chronic liver disease (7). The National Lipid Association (NLA) expert liver panel has stated that patients with chronic liver disease, nonalcoholic fatty liver disease and nonalcoholic steatohepatitis may safely receive statin therapy, supporting no scientific evidence to contraindicate statin use in patients with compensated cirrhosis (8). Literature has also indicated that use of statins in patients with hepatitis C infection and primary biliary cirrhosis is safe. Statins have not been shown to worsen the outcome in persons with chronically increased transaminase levels due to hepatitis B or C viruses (9). However, the incidence of discontinuations from statins due to hepatotoxicity was significantly higher in HBsAg-positive than in HBsAb-negative patients with coronary heart disease (10). Statins are contraindicated in patients with active liver disease or unexplained, persistent increases in liver function tests.
Except for cerivastatin, all statins have a very low systemic bioavailability owing to their extensive first-pass extraction by the liver. All statins, except for pravastatin, undergo microsomal metabolism by the cytochrome P450 (CYP) isoenzyme systems. Inhibition of CYP450 isoenzymes by concomitantly interacting drugs can lead to increased plasma concentrations of statins, resulting in myositis and rhabdomyolysis. Fibrates, warfarin, digoxin and mibefradil have been reported in patients with statin-associated rhabdomyolysis (11). Advanced age, diabetes, chronic renal insufficiency, and multiple medications are also associated with increased risks of statin-associated myopathy (9). Our patient, however, was not taking any drugs that interact with statins, nor was he ingesting alcohol. He did not have any other factors including hypokalemia, hypophosphatemia, hypo- or hyperthyroidism, and diuretics that could predispose him to the development of myopathy. No clinically important interactions have been observed between statins and other drugs used in cardiovascular diseases, such as propranolol, angiotensin-converting enzyme inhibitors, and thiazide diuretics (12).
About half of all statins currently available in clinical practice are biotransformed in the liver, primarily by the CYP450 3A4 system. Fluvastatin, unlike other statins, is metabolized primarily by the CYP2C9 enzyme in the liver, with CYP3A4 and CYP2C8 contributing to a lesser extent, and is therefore less subject to interactions than other statins (13). Thus, fluvastatin has a favorable adverse effect profile among the statins. As fluvastatin has the shortest elimination half-life of inactive metabolites among the statins and higher liver extraction, however, it results in high liver and peripheral tissue concentration, minimizing the systemic burden, instead potentiating hepatocellular injury in serious liver disease (6). In a population based cohort study (14), the highest risk of serious liver dysfunction among statins was associated with fluvastatin (hazard ratio 2.53, 95% confidence interval 1.84-3.47), which was significantly higher than that with simvastatin (hazard ratio 1.52, 95% confidence interval 1.38 to 1.66). With regard to this, our patient with liver cirrhosis was subject to serious adverse effects.
Among his medications, betaxolol is metabolized by CYP1A2 and CYP2D6 (15). Candesartan is primarily excreted as unchanged drug (75%) in the urine and feces with a smaller proportion (20%-25%) inactivated by CYP2C9. Entecavir and lactulose are not substrates for and do not inhibit or induce CYP isoenzymes. However, the major enzymes involved in glucuronidation and hydroxylation of NSAIDs are CYP2C9 (16). Therefore, we could not exclude the possibility that CYP2C9 enzyme in our patient was partially saturated with candesartan and aspirin or he had genetic variants in CYP2C9, which we could not identify unfortunately.
Statin-associated myopathies are dose-dependent and comparable among the various statins. Simvastatin is more potent than fluvastatin in lowering cholesterol. At doses of 20 mg/d, simvastatin and fluvastatin can reduce low-density lipoprotein by 30%-40% and 10%-20%, respectively. Lipophilic statins, including lovastatin, simvastatin, and cerivastatin, also have a greater potential for muscle toxicity than do hydrophilic statins including fluvastatin, atorvastatin, and pravastatin owing to their enhanced ability to penetrate myocytes. In our patient, rhabdomyolysis occurred after switching from a high- to a low-potency statin. It could be preliminarily explained in addition from a report that hepatic reactions associated with fluvastatin might be more common and more serious than those caused by other statins (17), especially in patients with hepatic dysfunction. In one third of severe hepatotoxicity associated with fluvastatin, it occurred after < 1 month of therapy.
Fluvastatin is an established treatment for hypercholesterolemia; its efficacy and safety have been documented in several clinical trials. Rhabdomyolysis associated with fluvastatin is very rare, with the adverse event reporting system of the U.S. Food and Drug Administration showing that only 1.7% of the incidents of statin-associated rhabdomyolysis were related to fluvastatin administration. However, our findings indicate that fluvastatin should be used with caution in patients with liver cirrhosis, especially with other medications metabolized with CYP2C9.
Figures and Tables
References
1. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001. 285:2486–2497.
2. Suh JW, Choi DJ, Chang HJ, Cho YS, Youn TJ, Chae IH, Kim KI, Kim CH, Kim HS, Oh BH, Park YB. HMG-CoA reductase inhibitor improves endothelial dysfunction in spontaneous hypertensive rats via down-regulation of caveolin-1 and activation of endothelial nitric oxide synthase. J Korean Med Sci. 2010. 25:16–23.
3. Corsini A, Jacobson TA, Ballantyne CM. Fluvastatin: clinical and safety profile. Drugs. 2004. 64:1305–1323.
4. Liberopoulos EN, Daskalopoulou SS, Mikhailidis DP, Wierzbicki AS, Elisaf MS. A review of the lipid-related effects of fluvastatin. Curr Med Res Opin. 2005. 21:231–244.
5. Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med. 2002. 346:539–540.
6. Akoglu H, Yilmaz R, Kirkpantur A, Arici M, Altun B, Turgan C. Combined organ failure with combination antihyperlipidemic treatment: a case of hepatic injury and acute renal failure. Ann Pharmacother. 2007. 41:143–147.
7. Russo MW, Jacobson IM. How to use statins in patients with chronic liver disease. Cleve Clin J Med. 2004. 71:58–62.
8. Cohen DE, Anania FA, Chalasani N. National Lipid Association Statin Safety Task Force Liver Expert Panel. An assessment of statin safety by hepatologists. Am J Cardiol. 2006. 97:77C–81C.
9. Pasternak RC, Smith SC Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. American College of Medicine, Ameican Heart Association, National Heart Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002. 40:567–572.
10. Zhang Q, Yang Z. Can statins be used safely in coronary heart disease patients of hepatitis B virus carriers? Int J Cardiol. 2011. 146:291.
11. Omar MA, Wilson JP. FDA adverse event reports on statin-associated rhabdomyolysis. Ann Pharmacother. 2002. 36:288–295.
12. Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation. 2004. 109:III50–III57.
13. Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther. 1999. 84:413–428.
14. Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010. 340:c2197.
15. Zateyshchikov DA, Minushkina LO, Brovkin AN, Savel'eva EG, Zateyshchikova AA, Manchaeva BB, Nikitin AG, Sidorenko BA, Nosikov VV. Association of CYP2D6 and ADRB1 genes with hypotensive and antichronotropic action of betaxolol in patients with arterial hypertension. Fundam Clin Pharmacol. 2007. 21:437–443.
16. Miners JO, Birkett DJ. Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol. 1998. 45:525–538.
17. Conforti A, Magro L, Moretti U, Scotto S, Motola D, Salvo F, Ros B, Leone R. Fluvastatin and hepatic reactions: a signal from spontaneous reporting in Italy. Drug Saf. 2006. 29:1163–1172.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download