Abstract
Campomelic dysplasia (CD; OMIM #114290), a rare form of congenital short-limbed dwarfism, is due to mutations in SOX9, a member of the SOX (SRY-related HMG box) gene family. Multiparous mother at 38 weeks' gestation delivered a 3,272 g baby boy with characteristic phenotypes including bowing of the lower limbs, a narrow thoracic cage, 11 pairs of ribs, hypoplastic scapulae, macrocephaly, flattened supraorbital ridges and nasal bridge, cleft palate, and micrognathia. He underwent a tracheostomy at the age of three months for severe laryngomalacia after a number of repeated hospitalizations due to respiratory problems and died at the age of four months from progressive respiratory failure. He was diagnosed as having CD based on a novel frameshift mutation (p.Gln458ArgfsX12) in the SOX9 gene, the mutation which has not yet been reported in Korea.
Campomelic dysplasia (CD; OMIM #114290) is a sporadic autosomal dominant disorder that results in skeletal and developmental abnormalities (1, 2). Its reported incidence is about 0.05-0.09 per 10,000 live births (3). Spranger and Maroteaux et al. (4) first described it fully and originally in 1971. CD is a frequently lethal skeletal dysplasia syndrome whose hallmark features include angular bowing and shortening of the long bones with pretibial skin dimpling, hypoplastic scapulae, missing pairs of ribs, a narrow thorax, and bilateral club feet. In addition, hydrocephalus, hydronephrosis, and congenital heart disease (ventriculoseptal defect, atrioseptal defect, aortic stenosis, and/or tetralogy of Fallot) are also present. Patients severely affected by CD usually die from respiratory distress during the neonatal period.
A secondary feature of CD is male-to-female sex reversal, which occurs in about two-thirds of patients with an XY karyotype. Like the sex reversal and the various skeletal symptoms, the bending of the long bones is not an obligatory feature and is absent in about 10% of cases, referred to as acampomelic CD (5). To our knowledge, the present case is the first reported case in Korea of a male with a novel SOX9 frameshift mutation.
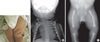
A baby boy was born to a gravida 2 mother with a vertex presentation following a full-term pregnancy in Asan Medical Center's neonatal intensive care unit (NICU) on August 20, 2008. Her first born had been stillborn and was reported to have no anomalies. An ultrasound study at 36 weeks of gestation during this second pregnancy demonstrated shortened long bones, club feet, and a large head. At birth, the one and five minute Apgar scores were 6 and 8, respectively. The boy's birth measurements were as follows: weight 3,272 g (25th percentile), length 46 cm (25th-50th percentile), and head circumference 37 cm (75th-90th percentile). His facial features included a flattened and prominent forehead, a flattened nasal bridge, and short palpebral fissures. Micrognathia and retrognathia were also present, as was a cleft in the soft palate. His ears were set abnormally low. Skeletal deformities included a small thoracic cage, short limbs, pretibial skin dimples on the left thigh (Fig. 1A), anterolateral femoral bowing, and clubbed feet. Laboratory investigations of the blood were normal. A chest radiograph showed a small bell-shaped thoracic cage and 11 pairs of ribs with mild T-L scoliosis and hypoplastic scapulae (Fig. 1B). The pelvic and lower limbs' radiography demonstrated a bowed femur, short fibulae, and no visible talus on either side (Fig. 1C). Pelvic sonography revealed bilateral complete hip dislocations with pseudoacetabular formation. Echocardiography showed a 6.4 mm secundum atrioseptal defect without other malformations. Cerebral ultrasonography on the fifth day showed a small cystic change from both germinal matrix hemorrhages. The brainstem auditory evoked potentials were normal. The patient's karyotype was 46XY with male external genitalia.
To screen for a mutation, we obtained informed consent from the parents for blood sampling. We isolated genomic DNA from peripheral blood using a QuickGene DNA kit (Fujifilm Life Science, Tokyo, Japan). To analyze the SOX9 gene's mutation, we performed PCR using eight sets of primers designed in the intronic flanking region and containing three exons referred to by GenBank accession number NT_010641.15. We performed DNA sequencing using the same primers used in PCR and a BigDye Terminatore V3.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA). Direct automated sequencing identified a novel frameshift mutation at nucleotide 1372 in exon 3 (Fig. 2). The patient carried both mutant and normal alleles, indicating that the mutation was heterozygous. Both parents declined genetic analysis.
After his discharge from NICU, the patient was hospitalized four times for treatments of pneumonia. He underwent a tracheostomy at the age of three months for severe laryngomalacia and died at the age of four months from progressive respiratory failure.
Characteristic features of CD are skeletal hypoplasias and anomalies affecting the face, head, scapulae, spine, pelvis, and upper and lower limbs. The head is macrocephalic with flattened face and nasal bridge, high forehead, low-set ears often with associated deafness, hypertelorism, long philtrum, small mouth, and micrognathia (6-9). The skeletal features are the most prominent characteristics of CD as presented in our case including anterior bowing of the tibia and characteristic pretibial skin dimples. The femurs are also mildly angulated, and talipes equinovarus and dislocation of the hips are usually present. Short fibulae, kyphoscoliosis, brachydactyly and clinodactyly are common. In addition, usually present are flat vertebrae (particularly at the cervical level), hypoplastic scapulae, and a small bell-shaped chest that's often slender with 11 pairs of ribs and a poorly mineralized sternum (7-9). After the critical first year, quality of life tends to improve, although most survived patients are known to be mentally retarded. The oldest reported survivor was a 17-yr-old who had an IQ of 45 (7, 9).
Most cases of CD are caused by heterozygous de novo mutations of the SOX9 gene at chromosome 17q 24.3-q25.1 (5, 10). A growing number of reports describes CD with the chromosome 17 rearrangement breakpoint located some distance from SOX9 (1, 10-12). SOX9 contains a 79-amino acid DNA-binding motif known as the high-mobility-group (HMG) domain, which recognizes typical SOX binding sequences and a second domain essential for its function, a proline/glutamine/serine-rich C-terminal transcription-activation domain (1, 13, 14). SOX9 is a transcription factor that plays a role in the expression of COL2A1, a major collagen gene, and anti-Müllerian hormone, which is secreted from the Sertoli cells for male sex differentiation (15, 16). The mutations cause a loss of either DNA binding or SOX9's transactivation function.
CD is a good model to illustrate how a single transcription factor can control the development of several organs. Both the skeletal dysplasia and the XY sex reversal in CD are caused by mutations in SOX9. All reported mutations in SOX9 can cause CD, and approximately 75% are associated with XY sex reversal; whereas no mutation in SOX9 has been associated with isolated sex reversal (17). The type and location of mutations in SOX9 have demonstrated no correlation with phenotype. Bernard et al. (18) demonstrated that cooperative dimerization of SOX9 was essential for activation of key chondrogenesis genes, but not for male gonadal development, which might explain why CD is not necessarily associated with XY sex reversal as shown in our case.
Four major classes of heterozygous SOX9 mutations cause CD: 1) amino acid substitutions in the HMG domain, 2) truncations or frameshifts that alter the C-terminus, 3) mutations at the splice junction, and 4) chromosomal translocations. All reported missense mutations lie in the HMG domain and affect DNA binding, frameshifts, and splice mutations that truncate SOX9's C-terminus, resulting in the loss of transactivation domains (17, 18). In the present case we identified, a novel frameshift mutation in codon 458 at nucleotide 1372. This mutation altered the transcription activation domain in the C-terminus, leading to a loss of SOX9's function.
To date, two cases of CD have been reported in Korea (19, 20). The first had multiple congenital anomalies (polysplenia, complex heart disease, bilaterally trilobed lungs, and a brain anomaly) and died immediately after delivery. The other was a case in which CD was identified in utero at 30 weeks of gestation whose pregnancy ended in abortion. Neither patient underwent a genetic analysis. Herein we report a novel de novo frameshift mutation (p.Gln458ArgfsX12) in the SOX9 gene of a Korean male with CD.
Figures and Tables
Fig. 1
Clinical features of a campomelic dysplasia patient. (A) Pretibial skin dimple (arrow) on the left thigh. (B) Chest radiography shows a bell-shaped, narrow thoracic cage, hypoplastic scapulae and mild T-L scoliosis. (C) Radiograph of bilateral legs shows anterolateral femoral bowing and short fibulae.
References
1. Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, Scherer G. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994. 79:1111–1120.
2. Kwok C, Weller PA, Guioli S, Foster JW, Mansour S, Zuffardi O, Punnett HH, Dominguez-Steglich MA, Brook JD, Young ID, Goodfellow PN, Schafer AJ. Mutations in SOX9, the gene responsible for Campomelic dysplasia and autosomal sex reversal. Am J Hum Genet. 1995. 57:1028–1036.
3. Stoll C, Dott B, Roth MP, Alembik Y. Birth prevalence rates of skeletal dysplasias. Clin Genet. 1989. 35:88–92.
4. Maroteaux P, Spranger J, Opitz JM, Kucera J, Lowry RB, Schimke RN, Kagan SM. The campomelic syndrome. Presse Med. 1971. 79:1157–1162.
5. Mansour S, Hall CM, Pembrey ME, Young ID. A clinical and genetic study of campomelic dysplasia. J Med Genet. 1995. 32:415–420.
6. Horton WA, Hecht JT. Behrman RE, Kliegman RM, Jenson HB, editors. The skeletal dysplasia. Textbook of Pediatrics. 2000. 16th ed. Philadelphia: WB Saunders Co;2113–2132.
7. Argaman Z, Hammerman CA, Kaplan M, Schimmel M, Rabinovich R, Tunnessen WW Jr. Picture of the month. Campomelic dysplasia. Am J Dis Child. 1993. 147:205–206.
8. Hall BD, Spranger JW. Campomelic dysplasia. Further elucidation of a distinct entity. Am J Dis Child. 1980. 134:285–289.
9. Jones KL. Smith's Recognizable Patterns of Human Malformation. 1997. 5th ed. Philadelphia: WB Saunders Co;344–345.
10. Foster JW, Dominguez-Steglich MA, Guioli S, Kowk C, Weller PA, Stevanović M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, Brook JD, Schafer AJ. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994. 372:525–530.
11. Offiah AC, Mansour S, McDowall S, Tolmie J, Sim P, Hall CM. Surviving campomelic dysplasia has the radiological features of the previously reported ischio-pubic-patella syndrome. J Med Genet. 2002. 39:e50.
12. Hill-Harfe KL, Kaplan L, Stalker HJ, Zori RT, Pop R, Scherer G, Wallace MR. Fine mapping of chromosome 17 translocation breakpoints > or = 900 Kb upstream of SOX9 in acampomelic campomelic dysplasia and a mild, familial skeletal dysplasia. Am J Hum Genet. 2005. 76:663–671.
13. Bianchi ME, Beltrame M. Flexing DNA: HMG-box proteins and their partners. Am J Hum Genet. 1998. 63:1573–1577.
14. McDowall S, Argentaro A, Ranganathan S, Weller P, Mertin S, Mansour S, Tolmie J, Harley V. Functional and structural studies of wild type SOX9 and mutations causing campomelic dysplasia. J Biol Chem. 1999. 274:24023–24030.
15. Bell DM, Leung KK, Wheatley SC, Ng Lj, Zhou S, Ling KW, Sham MH, Koopman P, Tam PP, Cheah KS. SOX9 directly regulates the type-II collagen gene. Nat Genet. 1997. 16:174–178.
16. De Santa Barbara P, Bonneaud N, Boizet B, Desclozeaux M, Moniot B, Sudbeck P, Scherer G, Poulat F, Berta P. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Müllerian hormone gene. Mol Cell Biol. 1998. 18:6653–6665.
17. Cameron FJ, Sinclair AH. Mutations in SRY and SOX9: testis-determining genes. Hum Mutat. 1997. 9:388–395.
18. Bernard P, Tang P, Liu S, Dewing P, Harley VR, Vilain E. Dimerization of SOX9 is required for chondrogenesis, but not for sex determination. Hum Mol Genet. 2003. 12:1755–1765.
19. Chung CQ, Bae HY, Kim DR, Park YH, Chung HS. A case of multiple congenital anomaly. Korean J Obstet Gynecol. 1992. 35:1407–1413.
20. Kim SK, Kim HC, Shin SJ, Lee MW, Lee YM, Cho JH, Choi YJ, Kwon KW. A case of fetal skeletal anomaly of Campomelic syndrome. Korean J Obstet Gynecol. 2000. 43:311–314.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download