Abstract
Pulmonary alveolar microlithiasis (PAM) is a rare disease with unknown etiology and pathogenesis. It is characterized by diffuse, innumerable, and minute calculi, called microlithiasis in the alveoli. More than half of reported cases are asymptomatic at the time of diagnosis. We describe the first case of PAM in Korea. A 19-yr-old man without respiratory symptoms presented with interstitial thickening on the chest radiograph. His chest high resolution CT scan showed diffusely scattered, ill defined tiny micronodules and interstitial thickening. Open lung biopsy confirmed the diagnosis of PAM. He was followed up for 6 months without treatment, and no progression was noticed.
Pulmonary alveolar microlithiasis (PAM) is a rare and chronic lung disease, characterized by the presence of small round bodies containing concentric calcareous lamellas in pulmonary alveoli (1-3). The disease could remain asymptomatic for decades, with subsequent occurrence of dyspnea, chest pain, and dry cough, and could result in respiratory failure (3). Half of the patients were asymptomatic at the time of diagnosis; therefore, most reported cases were diagnosed during a routine examination (1-3). Characteristic radiologic findings consist of bilateral innumerous calcified nodules, which might suggest the disease, and it is subsequently confirmed with a less invasive procedure, such as bronchoscopy (4). Most cases of PAM in Asia have been reported in Turkey, India, and Japan (1-3). However, no case has been reported in Korea. Here we reported on the first case of PAM in Korea, confirmed by open lung biopsy.
A 19-yr-old male exhibiting no respiratory symptoms visited our hospital on November 9, 2010 for evaluation of abnormalities noted on a chest radiogram of a routine health check for military service. He was a current smoker with a smoking history of 1.5 pack years. He had eight elder sisters, but no family history disclosing pulmonary disease. On admission, the patient had evidenced no abnormal physical findings, and laboratory data were also normal. Results of the pulmonary function test were also normal; an FVC of 4.48 liters (115% predicted), a normal FEV1 of 3.83 liters (107% predicted), and a FEV1/FVC ratio of 85%. Diffusion capacity study showed a DLCO of 33.4 mL/mmHg/min (126% predicted).
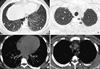
A chest radiogram taken on the day of his visit showed bilateral, diffuse, fine reticulonodular opacities in both lungs (Fig. 1). High resolution CT (HRCT) scan revealed scattered micronodules, interlobular septal thickening, subpleural interstitial thickening, and some areas of ground-glass opacity. Subpleural cystic changes and microcalcifications along the subpleural regions and some interlobar septa were also observed (Fig. 2).
Bronchoscopy was performed. Cell analysis of bronchoalveoloar lavage (BAL) fluid showed 80% in lymphocytes and 15% in monocytes. BAL fluid was negative for tuberculosis, malignancy, or microliths. Transbronchial lung biopsy showed a few alveolar tissues with multiple calcified nodules. Video-assisted thoracoscopic surgery (VATS) was performed for pathologic confirmation. Histological findings of VATS biopsy showed multiple round small calcified nodules in the visceral pleura and the alveolar wall, consistent with the diagnosis of PAM (Fig. 3).
The patient was followed up in out-patient clinic for 6 months without progression of the disease and had no treatment for PAM.
This is the first report on a case of PAM in Korea. PAM is a rare disease and has been reported with no particular geographic or racial distribution; however, Europe is the most prevalent area, followed by Asia, particularly Turkey, India, and Japan (1, 2). However, no case of PAM has been reported in Korea.
The etiology of the disease is still unknown. A genetic factor has been supposed because of familial occurrence (1, 2). Recently, a mutation in the SCL34A2 gene, which encodes a type IIb sodium dependent phosphate transporter, was suggested as a pathogenesis of this disease (5). CL34A2 is primarily expressed in alveolar type II cells responsible for surfactant production using phospholipids and plays a major role in homeostasis of inorganic phosphate (5). Therefore, dysfunction in CL34A2 can result in accumulation of phosphorus ion in alveolar space and is likely the cause of PAM. In this case, none of the family members of the patient had experienced any type of respiratory illness.
There was no sex difference in the rate of occurrence in reported cases and it was most frequently discovered from birth up to 40 yr of age (1-3). Due to the inability to certify clear etiological and pathogenic factors, a therapeutic approach is difficult, and there is currently no effective medical treatment for this disease and affected individuals may progress to respiratory failure. Therefore, lung transplantation should be considered in end stage PAM, where either severe respiratory failure or right heart failure is present (6-8).
Precise assessment of the history of alveolar microlithiasis is difficult. Therefore, in many cases, the diagnosis is made as an incidental radiographic film in symptomless patients (3). The chest radiograph usually revealed small innumerable, widespread, and calcified nodules, which tend to be greatest at the base (4, 9, 10). HRCT scan has aided in characterization of the imaging of the disease and can be useful in diagnosis (4, 9, 10). Ground-glass opacities due to volume averaging of tiny microliths are a common and major finding in the literature (4, 9, 10). Subpleural linear calcification and nodular fissural thickening are also a common finding (4, 9, 10). In this case, these findings were also observed on his chest CT. Although specific findings in HRCT scan could lead to diagnosis of PAM, other cases of diffuse pulmonary calcification should be considered for differential diagnosis, including metastatic pulmonary calcification, amyloidosis, and dendriform pulmonary ossification (11). However, clinician's awareness of typical HRCT findings of this disease could lead to diagnosis using less invasive procedures. Therefore, most recently reported cases of PAM were diagnosed by transbronchial biopsy and BAL (1). In this case, due to lack of experience with this disease, we performed open lung biopsy for diagnosis.
In conclusion, PAM is a rare disease that can present with no symptoms. Characteristic HRCT findings can aid in diagnosis of this disease using less invasive procedures.
Figures and Tables
Fig. 1
Chest radiogram (posteroanterior view) shows bilateral diffuse fine reticulonodular opacities in both lungs.
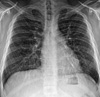
Fig. 2
Chest CT images of a patient with pulmonary alveolar microlithiasis. (A) HRCT image at the level of basal lung zone shows micronodules (white arrows), inter lobular septal thickening (black arrows), subpleural interstitial thickening (arrow heads), and some areas of ground- glass opacity (asterisk). (B) HRCT image at the level of apical lung zone shows micronodules (white arrows) and subpleural cystic changes (black arrows). (C, D) Mediastinal window scans show microcalcifications along the subpleural regions and some interlobar septa.
References
1. Castellana G, Lamorgese V. Pulmonary alveolar microlithiasis. World cases and review of the literature. Respiration. 2003. 70:549–555.
2. Lauta VM. Pulmonary alveolar microlithiasis: an overview of clinical and pathological features together with possible therapies. Respir Med. 2003. 97:1081–1085.
3. Mariotta S, Ricci A, Papale M, De Clementi F, Sposato B, Guidi L, Mannino F. Pulmonary alveolar microlithiasis: report on 576 cases published in the literature. Sarcoidosis Vasc Diffuse Lung Dis. 2004. 21:173–181.
4. Marchiori E, Gonçalves CM, Escuissato DL, Teixeira KI, Rodrigues R, Barreto MM, Esteves M. Pulmonary alveolar microlithiasis: high-resolution computed tomography findings in 10 patients. J Bras Pneumol. 2007. 33:552–557.
5. Corut A, Senyigit A, Ugur SA, Altin S, Ozcelik U, Calisir H, Yildirim Z, Gocmen A, Tolun A. Mutations in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular microlithiasis. Am J Hum Genet. 2006. 79:650–656.
6. Samano MN, Waisberg DR, Canzian M, Campos SV, Pêgo-Fernandes PM, Jatene FB. Lung transplantation for pulmonary alveolar microlithiasis: a case report. Clinics (Sao Paulo). 2010. 65:233–236.
7. Shigemura N, Bermudez C, Hattler BG, Johnson B, Crespo M, Pilewski J, Toyoda Y. Lung transplantation for pulmonary alveolar microlithiasis. J Thorac Cardiovasc Surg. 2009. 139:e50–e52.
8. Stamatis G, Zerkowski HR, Doetsch N, Greschuchna D, Konietzko N, Reidemeister JC. Sequential bilateral lung transplantation for pulmonary alveolar microlithiasis. Ann Thorac Surg. 1993. 56:972–975.
9. Gasparetto EL, Tazoniero P, Escuissato DL, Marchiori E, Frare E Silva RL, Sakamoto D. Pulmonary alveolar microlithiasis presenting with crazy-paving pattern on high resolution CT. Br J Radiol. 2004. 77:974–976.
10. Hoshino H, Koba H, Inomata S, Kurokawa K, Morita Y, Yoshida K, Akiba H, Abe S. Pulmonary alveolar microlithiasis: high-resolution CT and MR findings. J Comput Assist Tomogr. 1998. 22:245–248.
11. Chung MJ, Lee KS, Franquet T, Muller NL, Han J, Kwon OJ. Metabolic lung disease: imaging and histopathologic findings. Eur J Radiol. 2005. 54:233–245.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download