Abstract
Figures and Tables
Fig. 1

Fig. 2

Fig. 3
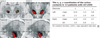
Fig. 4
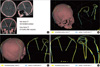
Fig. 5
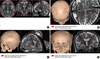
Table 1
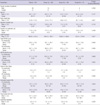
1For gender (discrete scale), the P value was estimated by chi-square test; For other variables (continuous scale), the P value was estimated by ANOVA (Analysis of Variances). *Daily levodopa equivalent dose (LEDD) was calculated for each antiparkinsonian medication by multiplying the total daily dosage of each drug by its potency relative to a standard levodopa preparation assigned at the value of 1. The following conversion factors were used: levodopa controlled release = 0.77, bromocriptine = 10, ropinirole = 20, pramipexole = 100, and pergolide = 100. One patient had a cardiac pacemaker for the sick sinus syndrome prior to STN DBS. Two patients had previous thalamotomy. ADL, Activities of Daily Life; LEDD, Daily levodopa equivalent dose; UPDRS, Unified Parkinson's Disease Rating Scale.
Table 2

All data was expressed as a mean ± standard deviation. *P < 0.05. The bold-faced P values were statistically significant (P < 0.005) after Bonferroni multiple comparison correction of 10 clinical outcomes; †significant different group among three groups or three repeated measured outcomes in the repeated measures ANOVA. 1, with DBS stimulation; 2, P value for within-factor of three repeated measured outcomes at the baseline and 6 and 12 months after bilateral STN stimulation; 3, P value for between-group-factor of three groups: I (both electrodes in the STN), II (only 1 electrode in the STN) and III (neither electrode in the STN); 4, P value for interaction between the within-factor and between-group-factor. Abbreviations as Table 1.
Table 3

All data was expressed as a mean ± standard deviation. *P < 0.05. The bold-faced p-values were statistically significant (P < 0.004) after Bonferroni multiple comparison correction of 12 UPDRS III subscores. †significant different group among three groups or three repeated measured outcomes in the repeated measures ANOVA with post-hoc tests. ‡The speech subscore at 6 months or at 12 months was significantly lower than that at baseline (paired t-test, P < 0.001 for 6 months vs baseline; P < 0.001 for 12 months vs baseline). 1, with DBS stimulation; 2, P value for within-factor of three repeated measured outcomes at the baseline and 6 months and 12 months after bilateral STN stimulation; 3, P value for between-group-factor of three groups: I (both electrodes in the STN), II (only 1 electrode in the STN) and III (neither electrode in the STN); 4, P value for interaction between the within-factor and between-group-factor effects.
Table 4
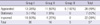
Mantel-Haenszel chi-square test for trend, chi-square value = 8.819 (degree of freedom = 1), P value = 0.003. Three groups were classified by the fused images of preoperative MRI and postoperative CT scans taken six months after surgery: group I, both electrodes in the STN; group II, only 1 electrode in the STN; group III; neither electrode in the STN. The assessment of speech improvement was based on the summation of the speech subscores of UPDRS part II and part III. All patients were evaluated at 12 months after bilateral STN stimulation.
Table 5

All data was expressed as a mean ± standard deviation. *P < 0.05. The bold-faced P values were statistically significant after Bonferroni multiple comparison correction (P < 0.005 for comparison between Baseline and On-DBS stimulation for 10 clinical outcomes; P < 0.01 for comparison between Baseline and Off-DBS stimulation for 4 clinical outcomes); †13 patients are in nil-LEDD and 44 patients are in the other group. 1, P value for within-factor of three repeated measured outcomes at the baseline and 6 months and 12 months after bilateral STN stimulation; 2, P value for between-group-factor of three groups: I (both electrodes in the STN), II (only 1 electrode in the STN) and III (neither electrode in the STN); 3, P value for interaction between the within-factor and between-group-factor. Abbreviations as Table 1.
Table 6
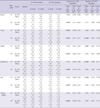
All data was expressed as a mean ± standard deviation. †13 patients are in nil-LEDD and 44 patients are in the other group. *P < 0.05. The bold-faced p-values were statistically significant (P < 0.004) after Bonferroni multiple comparison correction of 12 UPDRS III subscores. 1, P value for within-factor of three repeated measured outcomes at the baseline and 6 months and 24 months after bilateral STN stimulation; 2, P value for between-group-factor of three groups: I (both electrodes in the STN), II (only 1 electrode in the STN) and III (neither electrode in the STN); 3, P value for interaction between the within-factor and between-group-factor. Abbreviations as Table 1.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download