Abstract
The optimal duration of oral nucleos(t)ide analogue therapy for HBeAg negative chronic hepatitis B (CHB) has not been defined. The aim of this study was to investigate the clinical efficacy of 24-months course of lamivudine therapy in patients with HBeAg negative CHB in Korea. A total of 50 Korean patients with HBeAg negative CHB were prospectively enrolled. The patients received 100 mg/day of lamivudine orally for 24 months. Patients who showed complete response at 24 months to lamivudine therapy stopped treatment, and regular follow-up was done thereafter. The mean follow-up duration after cessation of therapy was 40.8±22.7 (range 12-96) months. The complete response rate at months 12 and 24 were 86.0% (43/50) and 86.0% (43/50), respectively, and the clinical breakthrough at months 12 and 24 were 4.0% (2/50) and 14.0% (7/50), respectively. The expected durability of responses at months 12, 24, and 36 after cessation of lamivudine therapy in 43 complete responders was 79.1%, 64.0%, and 56.9%, respectively. In conclusion, a 24-months course of lamivudine therapy shows high end-treatment response rate and substantial durability of initial response after cessation of therapy in HBeAg negative CHB patients in Korea.
Hepatitis B virus e antigen (HBeAg) negative chronic hepatitis B (CHB) represents a late phase of chronic hepatitis B virus (HBV) infection usually caused by precore or basic core promoter mutant viruses that are unable to produce HBeAg (1). HBeAg negative CHB is predominantly found in the Mediterranean region and Southern Europe, the middle East, and Asian countries, including Korea (2, 3). The prevalence of precore mutants varies geographically and is affected by the HBV genotype. In North Europe and North America, where genotype A predominates, HBeAg negative CHB is rare because cytosine at position 1858 (C-1858) precludes the selection of G1896A precore mutation. In Korea almost all patients with CHB have genotype C and both precore mutant and basic core promoter mutant are very common (3-5).
HBeAg negative CHB is a potentially severe and progressive form of liver disease with rare spontaneous remission and a high risk of progression to liver cirrhosis (6, 7). Moreover, it is harder to induce long-term sustained responsiveness after antiviral therapy in HBeAg negative CHB patients. A sustained-off therapy response rate has been reported at 10-30% in response to conventional interferon-α therapy (2, 8). Therefore, oral nucleos(t)ide analogues have been primarily considered, and used as, a therapeutic for HBeAg negative CHB.
Lamivudine is the first oral nucleoside analogue that has been used in the treatment of patients with HBeAg negative CHB. Therapy for 1 yr achieves complete (both biochemical and virological) responses at the end of therapy in approximately 2/3 of the patients (9-13). However, relapse after withdrawal of lamivudine is universal in patients who were treated for only 1 yr (14). Long-term maintenance therapy with lamivudine has been tried, but the response rate decreases with time because of the increasing emergence of lamivudine resistant mutants (9, 11, 12, 15). Most hepatitis B guidelines suggest that HBeAg negative CHB should be treated with oral nucleos(t)ide analogues for more than 1 yr, yet fail to address the duration of oral nucleos(t)ide analogues therapy beyond the first year (16-18). American Association for the Study of Liver Disease (AASLD) guideline suggest that treatment should be continued until the patient has achieved HBsAg clearance (16). However, long-term nucleos(t)ide analogue therapy has the potential risk of developing resistance or drug toxicity. In this study, we prospectively investigated the clinical efficacy of a 24-months course of lamivudine therapy in patients with HBeAg negative CHB.
This prospective study included a total of 50 patients with HBeAg negative CHB who started lamivudine therapy between December 1997 and December 2004 at Gangnam Severance Hospital in Korea. Over the course of 24 months, 100 mg of lamivudine was administered to patients daily. Patients who showed complete response at 24 months were removed from lamivudine therapy and had regular follow-up. Lamivudine therapy continued in patients who did not show complete response at 24 months. Patient followed-up consisted of physical examination, a routine laboratory test at least every 3 months, and an HBV DNA test at least every 6 months to check for any breakthrough. Complete response was defined as both normalization of alanine aminotransferase (ALT) and undetectability of HBV DNA (<0.5 pg/mL) according to hybrid capture assay.
Inclusion criteria was as follows: 1) Hepatitis B surface antigen (HBsAg) positive and HBeAg negative for longer than 6 months; 2) ALT elevation >1.5 of the upper limit of normal levels and HBV DNA positive within one month of the study; 3) No history of previous antiviral therapy; and 4) No interferon-α treatment within 18 months before the enrollment. No patients were positive to anti-hepatitis C virus (HCV) antibody (Ab) or anti-human immunodeficiency virus (HIV) Ab. Patients who had decompensated liver disease or hepatocellular carcinoma were excluded. This study was approved by the institutional review board (IRB approval number: 3-2007-0060) at Gangnam Severance Hospital (Seoul, Korea), and all participants gave written, informed consent.
HBsAg and HBeAg/Ab were determined by enzyme immunoassay (Dade Behring, Marburg, Germany). HBV DNA was measured by Digene hybrid capture assay (Digene Diagnostics, Beltsville, MD, USA) with a lower limit of 0.5 pg/mL. The antibody against HCV was detected by a third-generation enzyme-linked immunosorbent assay (Korea Greencross, Yongin, Korea).
All data were analyzed using the statistical software SPSS (version 11.0, SPSS Inc., Chicago, IL, USA). Quantitative variables are expressed as mean±standard deviation. Data were analyzed using Student's t-test, Mann-Whitney U test, and the chi-square test. The Kaplan-Meier method was used to estimate the durability of response after stopping lamivudine therapy. Data were considered to be statistically significant with P<0.05.
The baseline characteristics of the 50 patients are presented in Table 1. There were 39 men and 11 women, with a mean age of 43±11 yr. Median serum AST and ALT levels were 152 IU/L and 239 IU/L, respectively. All patients were HBV DNA positive (>0.5 pg/mL). Fifteen patients (30%) were clinically diagnosed with liver cirrhosis. Twelve patients (24%) had a history of previous interferon-α therapy. Thirty six patients (72%) had a family history of HBV infection. The total follow-up duration from the beginning of lamivudine therapy was 64.7±22.5 (range, 36-120) months.
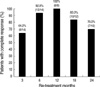
Complete response was defined as having both ALT normalization and HBV DNA undetectability (<0.5 pg/mL) as measured by the hybrid capture assay. The complete response rate at 3, 6, 12, and 24 months of lamivudine therapy was 64.0% (32 of 50 patients), 90.0% (45 of 50), 86.0% (43 of 50), and 86.0% (43 of 50), respectively (Fig. 1). Cumulative clinical breakthrough (HBV DNA positive conversion with ALT re-elevation) occurred in 4.0% (2 of 50 patients) at 12 months, and 14.0% (7 of 50 patients) at 24 months of therapy (Fig. 1). Lamivudine therapy was stopped in 43 patients who showed complete response at the end of 24 months. Among the 7 patients who did not show complete response at the end of 24 months, 2 patients continued lamivudine therapy and the other 5 patients switched to adefovir therapy.
The 43 patients who showed complete response without evidence of clinical breakthrough at 24 months of lamivudine therapy were removed from treatment and regular follow-up was done thereafter. In these patients, the mean duration of follow-up after cessation of lamivudine therapy was 40.8±22.7 (range 12-96) months. The number of patients at follow up months 12, 24, 36, 48, and 60 were 33, 18, 14, 8, and 5, respectively. The expected durability of response after cessation of 24 months-course lamivudine therapy was calculated by the Kaplan-Meier method, and the results at months 12, 24, 36, 48, and 60 of follow-up were 79.1%, 64.0%, 56.9%, 47.4%, and 47.4%, respectively (Fig. 2). There were no episodes of hepatic decompensation in relapsed patients after withdrawal of lamivudine. When comparing the characteristics of those who have relapsed and those who have not relapsed, the proportion of liver cirrhosis patients was significantly higher in relapsers than non-relapsers (44% vs. 16%, P=0.040). Meanwhile, there was no difference in age, sex, baseline ALT level, previous interferon treatment history, family history of hepatitis B, or the follow-up duration after cessation of lamivudine therapy between the two groups (Table 2).
Fourteen patients among 18 patients who relapsed after cessation of lamivudine therapy were retreated with lamivudine. Complete response rates at 3, 6, 12, 18, and 24 months of lamivudine re-treatment were 64.2% (9 of 14 patients), 92.8% (13 of 14 patients), 100.0% (14 of 14 patients), 83.3% (10 of 12 patients), 70.0% (7 of 10 patients), respectively (Fig. 3). Clinical breakthrough (HBV DNA positive conversion with ALT re-elevation) occurred in 30.0% of patients during the second year of the re-treatment period (Fig. 3). The flow chart of the 24-months course of lamivudine therapy in HBeAg negative CHB patients is illustrated in Fig. 4.
Lamivudine has been widely used as an initial antiviral agent for the treatment of patients with chronic hepatitis B. Although there have been recent developments of anti-viral agents with a lower probability of viral resistance, such as adefovir or entecavir, lamivudine is still being widely used due to relatively cheap cost, rapid action, and proven safety for long-term use. Lamivudine monotherapy has been shown to benefit patients with HBeAg positive- or HBeAg negative-CHB (12, 19). For HBeAg positive CHB, HBeAg loss or seroconversion has been used as an indicator for considering when to stop oral antiviral therapy (16-18). According to AASLD practice guidelines, lamivudine therapy should be continued at least 6 months after the achievement of HBeAg seroconversion (16).
However, there is no indicator to determine treatment duration in HBeAg negative CHB patients. The optimal duration of treatment in HBeAg negative CHB is unknown. One year treatment with lamivudine in HBeAg negative CHB patients resulted in HBV DNA suppression to undetectable levels by PCR in 60 to 70% of patients (9-13). Most patients relapsed after withdrawal of lamivudine after 1 yr of treatment (14). This result supports the rationale that most practice guidelines recommend prolonged oral nucleos(t)ide analogue treatment for a period exceeding 1 yr in patients with HBeAg negative CHB (16-18).
Several prolonged antiviral therapies, for periods longer than 1 yr, have been tried in HBeAg negative CHB patients. Virological and biochemical response rates peaked at 12 months and decreased thereafter (9-13). It was reported that virological remission rates at years 1, 2, and 3 were 77%, 52%, and 42%, respectively, in Greek patients with HBeAg negative CHB. HBV genotype D is predominant in Greece, and almost all patients had HBV with precore stop codon mutation (11). Another study conducted in Greece reported that the virological response rate during the second year of lamivudine therapy in HBeAg negative patients was 34% (12). Long-term therapy with lamivudine in HBeAg negative CHB patients in the Mediterranean region, has been associated with increasing rates of virological breakthrough due to the selection of mutants of the YMDD motif of HBV DNA polymerase gene (rtM204I/V±rtL180M) (9, 11, 12, 15). Therefore, the decision to continue oral nucleos(t)ide therapy beyond 1 yr in HBeAg negative CHB should weigh the likelihood of benefit against the risk of developing resistance or drug toxicity (17).
There are two intriguing reports that confine the duration of antiviral therapy to two years in HBeAg negative CHB patients (20, 21). A study with a limited number of patients conducted in Spain, in which most patients had genotype A or D, showed that the response rates at years 1 and 2 of limivudine therapy were 81% and 69% respectively (20). Another study conducted in Canada reported that HBV DNA undetectability rates at years 1 and 2 of treatment were 92% and 86%, respectively, according to the hybrid capture assay during the 2-yr course of lamivudine therapy in 50 Chinese patients with HBeAg negative CHB. Most patients in the study had the HBV genotype C (21). The expected durability rates of virological response at 6, 12, and 18 months were 70%, 50%, and 50%, respectively (21).
Our results showed that the initial response rate at years 1 and 2 of lamivudine therapy was 86.0% and 86.0%, respectively, which compare well with those of a previous study by Fung et al. (21). Unlike previous reports in the Mediterranean region that show initial response rates during the first year of therapy dramatically fall during the second year, our study indicated that the initial response rate of the first year nearly maintained until the end of the second year of lamivudine treatment in Korean patients. Furthermore, our data indicated that the emergence rates of clinical breakthrough due to viral resistance during years 1 and 2 of lamivudine therapy were 4.0% and 14.0%, respectively, a rate lower than those reported in Southern Europe (9, 11, 12, 15). Lok et al. (22) reported that lamivudine resistant mutants were detected at similar rates in both patients with HBeAg positive CHB and those with HBeAg negative CHB. However, previous reports conducted in Asia, including Hong Kong and Korea, reported that the viral breakthrough rate was lower in HBeAg negative CHB patients than HBeAg positive CHB patients during long-term lamivudine therapy (23-25).
The differences of virological factors, including HBV genotype and the prevalence of precore or core promoter mutations, between Southern Europeans and Koreans may contribute to a difference in responsiveness to anti-viral therapy in HBeAg negative CHB. Although we did not analyze HBV genotypes or precore/core promoter mutations in this study, previous reports demonstrate that almost all Korean patients with chronic hepatitis B have genotype C and both precore mutant and basic core promoter mutants are very common in HBeAg negative CHB patients in Korea (3-5). Lamivudine therapy resulted in transient reversion to wild-type HBV from precore/core promoter mutants in Korea (26), but these changes were not observed in a long-term study of Greek patients treated with lamivudine (9). As the precore and core promoter mutants decrease or abolish HBeAg excretion and increase replication capacity (27, 28), the transient reversion to wild type precore/core promoter HBV, during lamivudine therapy in Korean HBeAg negative CHB patients, may delay the emergence of lamivudine-resistant mutant HBV in Korea. However, further studies will be needed to investigate the mechanism of the low rate of lamivudine resistance in Korean patients with HBeAg negative CHB.
Our data showed that the expected off-treament durability at months 12, 24, and 36 of follow-up in complete responders after a 24-months course of lamivudine therapy was 79.1%, 64.0%, and 56.9%, respectively. Most patients who relapsed after cessation of lamivudine therapy occurred within 24 months and then reached a plateau after 48 months of withdrawal from lamivudine therapy. Our data indicate that a 24-months course of lamivudine therapy could induce long-term remission in approximately half of the patients with HBeAg negative CHB in Korea, suggesting a 24-months course of lamivudine therapy could be a feasible option as a first line therapeutic strategy in the treatment of HBeAg negative CHB in Korean patients. When we compared the characteristics of relapsers and non-relapsers, the proportion of liver cirrhosis was significantly higher in relapsers than non-relapsers, suggesting cirrhotic patients with HBeAg negative CHB may require prolonged treatment (16-18). Of the patients who relapsed after withdrawal from lamivudine, none developed hepatic decompensation. Those patients who relapsed after withdrawal from lamivudine therapy were re-treated. Interestingly, the patient response to re-treatment was substantial. Nearly all patients showed ALT normalization with HBV DNA undetectability at 1 yr after re-treatment with lamivudine.
Our study had several limitations. The number of patients included was relatively small, and HBV DNA was measured by the hybrid capture assay, which is less sensitive than a PCR-based assay. However, those limitations could not be overcome in this study because the study was designed and started as a long-term prospective study from the late 1990s when HBV DNA quantitation by PCR was not routinely used in clinical setting. Based upon our data, a larger scaled study adopting more sensitive HBV DNA quantitation method would be necessary in the future.
In conclusion, A 24-months course of lamivudine therapy in Korean patients shows a significant initial response rate, and long-term remission can be maintained in about half of complete responders at 24-months after withdrawal of lamivudine. Moreover, lamivudine re-treatment is still effective in the viral suppression in relapsers after cessation of lamivudine therapy. Therefore a 24-months course of lamivudine therapy may be a feasible option as a first line therapeutic strategy in the treatment of HBeAg negative CHB in Korean patients.
Figures and Tables
Fig. 1
Complete response rate (hatched bar) and breakthrough rate (black bar) during a 24 months-course of lamivudine therapy in HBeAg negative CHB patients. Complete response was defined as having both ALT normalization and HBV DNA undetectability (<0.5 pg/mL) as measured by the hybrid capture assay. Clinical breakthrough was defined as HBV DNA positive conversion with ALT re-elevation.

Fig. 2
Expected durability of response after cessation of lamivudine in 43 patients who showed complete response at 24 months of lamivudine therapy (Kaplan-Meier analysis). Durability of response rates at 1, 2, and 3 yr are 79.1%, 64.0%, and 56.9%, respectively.

Fig. 3
Complete response during re-treatment in patients who relapsed after stopping lamivudine therapy. Complete response rate at 12 and 24 months of re-treatment was 100% and 70.0%, respectively.
References
1. Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2001. 34:617–624.

2. Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000--summary of a workshop. Gastroenterology. 2001. 120:1828–1853.

3. Yoo BC, Park JW, Kim HJ, Lee DH, Cha YJ, Park SM. Precore and core promoter mutations of hepatitis B virus and hepatitis B e antigen-negative chronic hepatitis B in Korea. J Hepatol. 2003. 38:98–103.

4. Song BC, Cui XJ, Kim H. Hepatitis B virus genotypes in Korea: an endemic area of hepatitis B virus infection. Intervirology. 2005. 48:133–137.

5. Lee JM, Ahn SH, Chang HY, Shin JE, Kim DY, Sim MK, Hong SP, Chung HJ, Kim SO, Han KH, Chon CY, Moon YM. Reappraisal of HBV genotypes and clinical significance in Koreans using MALDI-TOF mass spectrometry. Korean J Hepatol. 2004. 10:260–270.
6. Bonino F, Rosina F, Rizzetto M, Rizzi R, Chiaberge E, Tardanico R, Callea F, Verme G. Chronic hepatitis in HBsAg carriers with serum HBV-DNA and anti-HBe. Gastroenterology. 1986. 90:1268–1273.

7. Brunetto MR, Giarin MM, Oliveri F, Chiaberge E, Baldi M, Alfarano A, Serra A, Saracco G, Verme G, Will H. Wild-type and e antigen-minus hepatitis B viruses and course of chronic hepatitis. Proc Natl Acad Sci U S A. 1991. 88:4186–4190.

8. Hadziyannis SJ, Papatheodoridis GV, Vassilopoulos D. Treatment of HBeAg-negative chronic hepatitis B. Semin Liver Dis. 2003. 23:81–88.

9. Hadziyannis SJ, Papatheodoridis GV, Dimou E, Laras A, Papaioannou C. Efficacy of long-term lamivudine monotherapy in patients with hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2000. 32:847–851.

10. Rizzetto M, Volpes R, Smedile A. Response of pre-core mutant chronic hepatitis B infection to lamivudine. J Med Virol. 2000. 61:398–402.

11. Papatheodoridis GV, Dimou E, Laras A, Papadimitropoulos V, Hadziyannis SJ. Course of virologic breakthroughs under long-term lamivudine in HBeAg-negative precore mutant HBV liver disease. Hepatology. 2002. 36:219–226.

12. Papatheodoridis GV, Dimou E, Dimakopoulos K, Manolakopoulos S, Rapti I, Kitis G, Tzourmakliotis D, Manesis E, Hadziyannis SJ. Outcome of hepatitis B e antigen-negative chronic hepatitis B on long-term nucleos(t)ide analog therapy starting with lamivudine. Hepatology. 2005. 42:121–129.

13. Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A, Colonno R, Fernandes L. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006. 354:1011–1020.

14. Santantonio T, Mazzola M, Iacovazzi T, Miglietta A, Guastadisegni A, Pastore G. Long-term follow-up of patients with anti-HBe/HBV DNA-positive chronic hepatitis B treated for 12 months with lamivudine. J Hepatol. 2000. 32:300–306.

15. Manolakopoulos S, Karatapanis S, Elefsiniotis J, Mathou N, Vlachogiannakos J, Iliadou E, Kougioumtzan A, Economou M, Triantos C, Tzourmakliotis D, Avgerinos A. Clinical course of lamivudine monotherapy in patients with decompensated cirrhosis due to HBeAg negative chronic HBV infection. Am J Gastroenterol. 2004. 99:57–63.

17. de Franchis R, Hadengue A, Lau G, Lavanchy D, Lok A, McIntyre N, Mele A, Paumgartner G, Pietrangelo A, Rodes J, Rosenberg W, Valla D. EASL International Consensus Conference on Hepatitis B. 13-14 September, 2002 Geneva, Switzerland. Consensus statement (long version). J Hepatol. 2003. 39:Suppl 1. S3–S25.
18. Liaw YF, Leung N, Guan R, Lau GK, Merican I, McCaughan G, Gane E, Kao JH, Omata M. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update. Liver Int. 2005. 25:472–489.

19. Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003. 124:105–117.

20. Buti M, Cotrina M, Jardi R, de Castro EC, Rodriguez-Frias F, Sanchez-Avila F, Esteban R, Guardia J. Two years of lamivudine therapy in anti-HBe-positive patients with chronic hepatitis B. J Viral Hepat. 2001. 8:270–275.

21. Fung SK, Wong F, Hussain M, Lok AS. Sustained response after a 2-yr course of lamivudine treatment of hepatitis B e antigen-negative chronic hepatitis B. J Viral Hepat. 2004. 11:432–438.
22. Lok AS, Hussain M, Cursano C, Margotti M, Gramenzi A, Grazi GL, Jovine E, Benardi M, Andreone P. Evolution of hepatitis B virus polymerase gene mutations in hepatitis B e antigen-negative patients receiving lamivudine therapy. Hepatology. 2000. 32:1145–1153.

23. Chang YS, Yim JY, Cho NY, Choi CW, Baek SJ, Ahn SH, Choi DW, Kwon YD, Kim SS, Kwon OS, Kim JH, Yeon JE, Song JW, Byun KS, Lee CH. Viral breakthrough in HBeAg-negative chronic hepatitis B patients receiving lamivudine therapy. Korean J Hepatol. 2002. 8:397–404.
24. Shin JW, Chung YH, Choi MH, Kim JA, Ryu SH, Jang MK, Kim IS, Park NH, Lee HC, Lee YS, Suh DJ. Precore stop codon mutation of hepatitis B virus is associated with low breakthrough rate following long-term lamivudine therapy. J Gastroenterol Hepatol. 2005. 20:844–849.

25. Lau DT, Khokhar MF, Doo E, Ghany MG, Herion D, Park Y, Kleiner DE, Schmid P, Condreay LD, Gauthier J, Kuhns MC, Liang TJ, Hoofnagle JH. Long-term therapy of chronic hepatitis B with lamivudine. Hepatology. 2000. 32:828–834.
26. Cho SW, Hahm KB, Kim JH. Reversion from precore/core promoter mutants to wild-type hepatitis B virus during the course of lamivudine therapy. Hepatology. 2000. 32:1163–1169.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download