Abstract
Cardiac resynchronization therapy (CRT) has been proven its value in adult patients with congestive heart failure of low ejection fraction and wide QRS duration. Contrast to adult patients, CRT has been rarely applied for young patients. We report on a 9-yr-old boy with progressive left ventricular (LV) dilatation and dysfunction following chronic VVI pacemaker therapy for congenital complete atrioventricular block associated with maternal anti-SSA/Ro and SSB/La antibody. His LV dysfunction was improved after epicardially established CRT.
Right ventricular based pacing (RVP) has been standard therapy for children with advanced second-degree and complete atrioventricular (AV) block during the last several decades. Nevertheless, congestive heart failure (CHF) and dilated cardiomyopathy (DCM) may rarely occur in children undergoing chronic RVP systems (1, 2).
There are numerous clinical trials on CRT that show the effectiveness of it when dealing with or treating adult patients with refractory CHF and DCM (3, 4). However, most reports on CRT and its effectiveness have paid attention to adult patients rather than child patients. Not many studies on CRT with young children have been conducted. Some researches on the issue that were carried out only dealt with heterogeneous populations that are not capable of being generalized (5-7). We present a case of progressive left ventricular (LV) dysfunction following chronic right ventricular (RV) epicardial pacemaker for congenital complete AV block whose LV dysfunction was improved after CRT.
A 9-yr-old boy was transferred to our hospital because of progressive LV dysfunction on March 14, 2007. He was delivered by Caesarean section at 39 weeks of gestation and his birth weight was 3.2 kg. After birth, bradycardia due to congenital heart block was noted. At the age of 3 days, a permanent VVI pacemaker was implanted due to a resting heart rate of 50-60 beats/min, which was accompanied by LV dilatation and low-LV ejection fraction (EF) of 41%. After VVI pacing, LV dilatation and dysfunction had been improved.
At the age of 9 yr, LV dilatation and dysfunction were detected. He was referred to our outpatient department for further management of LV dysfunction. When he first visited our institution, he had not complained specific symptoms. The New York Heart Association (NYHA) class was II or I, occasionally. The cardiac examination revealed regular heart beat without murmur. The liver was impalpable.
Electrocardiogram showed VVI paced wide QRS complex of 164 msec with left bundle branch block (LBBB) pattern of QRS morphology and superior axis at frontal plane. Test weaning of ventricular pacing revealed intrinsic rhythm of complete heart block with wide QRS escape beats (Fig. 1A, B).
Echocardiography revealed severe LV dilation with paradoxical septal motion. LV EF measured by the biplane Simpson method was 25.1% (Fig. 2A). Dyskinesia of interventricular septum was also found (Fig. 3A). There was significant intraventricular dyssynchrony as follows: septal to lateral delay was 136 msec and all segment delay measured by tissue synchrony image (TSI) was 219 msec.
Chest radiography showed a cardiomegaly, of which cardiothoracic (CT) ratio was 0.61 (Fig. 4A). The level of plasma B-type natriuretic peptide (BNP) was 111 pg/mL. Screening for fluorescent antinuclear antibodies (FANA) and anti double-stranded deoxyribonucleic acid (ds-DNA) antibodies was negative. Both anti-SSA/Ro and anti-SSB/La antibodies were also negative. When we have examined his mother, the results were as follows: FANA 1:40, anti ds-DNA antibodies 6.0 and anti-SSA/Ro and SSB/La antibodies (+).
We started to prescribe enalapril to him. Because LV EF remained below 30% on echocardiography in spite of medical treatment for 1 yr, we decided to use CRT in an attempt to improve cardiac function and acquire the reversed remodeling.
He received an epicardial biventricular pacing system with bipolar leads (InSync® III model 8042, Medtronic Inc., Minneapolis, MN, USA) with DDD mode via median sternotomy. The epicardial bipolar atrial lead and the bipolar ventricular lead were placed on the right atrial (RA) appendage and the RV apex, respectively. The left lead was placed on the LV posteriolateral wall, between the posterior descending artery and the branches of left circumflex artery. In choosing pacing sites, in order for optimal synchronization in LV walls, we considered TSI by intraoperative transesophageal echocardiography and arterial pressure monitoring that brought about minimal dyssynchrony and maximal arterial pressure. The pacing thresholds of RA and LV were 1.0 V and 1.5 V respectively with 0.4 msec pacing width. Minimum intraventricular dyssynchrony was achieved with the setting of LV earlier by VV delay 4 msec.
Twelve months after implantation, electrocardiogram showed atrial-sensing and ventricular-pacing QRS complex of 148 msec (Fig. 1C). Echocardiographic evaluation demonstrated improved myocardial synchronicity and performance (Fig. 3B). LV EF was 59.3% (Fig. 2B). Septal to LV lateral delay decreased from 136 msec to -10 msec on TSI. In addition, the heart size was decreased as well. Chest radiography showed a CT ratio of 0.51 (Fig. 4B). Plasma BNP level decreased to 32 pg/mL.
Now, 18 months later from the implantation, he shows the improved exercise tolerance. He has been followed up at the outpatient department and observed on a regular basis. Currently he is in a treatment of the combination therapy using enalapril and carvedilol.
Prolonged ventricular dyssynchrony induced by long-term RVP is associated with deleterious LV remodeling, which are LV dilatation and LV asymmetrical hypertrophy (2, 8, 9). A recent small series study documented that CRT was effective in improving LV function in young patients with RVP induced cardiomyopathy (10, 11). Indications for CRT in the pediatric population are undefined. Very few children with idiopathic DCM meet the published criteria found predictive of a positive response in adult subjects (LBBB, LV dysfunction [EF <35%], and prolonged QRS duration [>125 msec]) (3, 4, 7). RVP induced cardiomyopathy seems to be one consistent subset of children and adolescent patients that seems eligible for CRT.
In this case with congenital heart block, RV paced QRS duration was prolonged (164 msec) and LV dysfunction was obvious (LV EF=25.1%). Although the heart failure was not severe, the LV dysfunction in this patient was considered to be caused by chronic RV pacing and intraventricular dyssynchrony. Because this patient had already VVI pacemaker, the pacemaker was upgraded to CRT. The underlying disease and life expectancy in children are different from those in adult, current indication for CRT in adult patients may not be strictly applicable to children.
Janousek et al. (5) described the use of biventricular pacing in one infant with congenital complete AV block and DCM that had been previously paced for complete AV block. Strieper et al. (6) reported an improved clinical status and LV EF following CRT in four of five patients previously paced for surgical complete AV block. In some study of resynchronization therapy in pediatric patients (7, 12), the authors reported the patients with AV block who exhibited an average improvement in LV EF. Our patient showed an improving LV EF and septal to LV lateral delay on TSI.
Similar to the improvement in LV function, our patient exhibited reduction in the plasma BNP level. The change of BNP has rarely been described in pediatric patients with dilated cardiomyopathy undergoing CRT (13). In adult patients, BNP levels are deemed as a useful index to reflect different degrees of LV reverse remodeling (14).
Meanwhile, insufficient shortening of QRS complex seems to be associated with intrinsic intraventricular conduction delay.
Udink ten Cate et al. (15) reported anti SSA/Ro and anti SSB/La antibodies were one of the risk factors for development of DCM in patients with congenital complete AV block. Even though our patient's anti SSA/Ro and anti SSB/La antibodies were negative, his maternal anti SSA/Ro and anti SSB/La antibodies were positive. Maternal antibody may result in not only the damage of the conduction tissue but also contracting myocardium of the fetal heart.
Direct association between RVP and development of severe DCM and overt heart failure in the young has not yet been demonstrated in Korea. This case is expected to play a significant role in RVP induced electromechanical dyssynchrony in the development of LV dysfunction irrespective of AV block etiology. Prospective and randomized studies are warranted to define the indication for pediatric CRT. And long term follow-up studies are also needed to determine not only beneficial effects and but also CRT-related late adverse effects in young children.
In summary, despite early institution of cardiac pacing, some patients with congenital AV block develop LV cardiomyopathy. Patients with congenital AV block have to be closely monitored not only their heart rate and rhythm but also ventricular function even after pacemaker implantation. Upgrading to CRT by biventricular pacing should be considered in the patient who has progressive LV dysfunction after RVP.
Figures and Tables
Fig. 1
Electrocardiographic findings before and after implantation. (A) Electrocardiogram showed complete heart block and wide QRS intrinsic escape rhythm with normal QRS axis. (B) VVI paced rhythm had wide QRS complex of 164 msec with superior axis. (C) Twelve months after implantation, electrocardiogram demonstrated atrial-sensing and ventricular-pacing QRS complex of 148 msec.

Fig. 2
M mode echocardiography revealed the improvement of left ventricular dilatation and fractional shortening. Panel (A) shows pre-cardiac resynchronization therapy (CRT) and panel (B) shows post-CRT images.
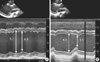
Fig. 3
Echocardiographic findings before and after implantation. (A) Tissue strain image at 4 chamber view shows inhomogenous left ventricular (LV) peak global strain (arrow) and markedly decreased septal strain. (B) Twelve months after implantation, echocardiography demonstrated improving LV contractility and rather homogenous LV peak global strain (arrow) than before.
References
1. Moak JP, Barron KS, Hougen TJ, Wiles HB, Balaji S, Sreeram N, Cohen MH, Nordenberg A, Van Hare GF, Friedman RA, Perez M, Cecchin F, Schneider DS, Nehgme RA, Buyon JP. Congenital heart block: development of late-onset cardiomyopathy, a previously underappreciated sequela. J Am Coll Cardiol. 2001. 37:238–242.

2. Thambo JB, Bordachar P, Garrigue S, Lafitte S, Sanders P, Reuter S, Girardot R, Crepin D, Reant P, Roudaut R, Jais P, Haissaguerre M, Clementy J, Jimenez M. Detrimental ventricular remodeling in patients with congenital complete heart block and chronic right ventricular apical pacing. Circulation. 2004. 110:3766–3772.

3. Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, Garrigue S, Kappenberger L, Haywood GA, Santini M, Bailleul C, Daubert JC. Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001. 344:873–880.

4. Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Messenger J. MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002. 346:1845–1853.

5. Janousek J, Tomek V, Chaloupecky V, Gebauer RA. Dilated cardiomyopathy associated with dual-chamber pacing in infants: improvement through either left ventricular cardiac resynchronization or programming the pacemaker off allowing intrinsic normal conduction. J Cardiovasc Electrophysiol. 2004. 15:470–474.

6. Strieper M, Karpawich P, Frias P, Gooden K, Ketchum D, Fyfe D, Campbell R. Initial experience with cardiac resynchronization therapy for ventricular dysfunction in young patients with surgically operated congenital heart disease. Am J Cardiol. 2004. 94:1352–1354.

7. Dubin AM, Janousek J, Rhee E, Strieper M, Cecchin F, Law IH, Shannon KM, Temple J, Rosenthal E, Zimmerman FJ, Davis A, Karpawich PP, Al Ahmad A, Vetter VL, Kertesz NJ, Shah M, Snyder C, Stephenson E, Emmel M, Sanatani S, Kanter R, Batra A, Collins KK. Resynchronization therapy in pediatric and congenital heart disease patients: an international multicenter study. J Am Coll Cardiol. 2005. 46:2277–2283.
8. Lee MY. Cardiac resynchronization therapy: biventricular pacing. Korean Circ J. 2006. 36:329–336.

9. Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, Sharma A. Dual Chamber and VVI Implantable Defibrillator Trial Investigators. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002. 288:3115–3123.
10. Moak JP, Hasbani K, Ramwell C, Freedenberg V, Berger JT, Dirusso G, Callahan P. Dilated cardiomyopathy following right ventricular pacing for AV block in young patients: resolution after upgrading to biventricular pacing systems. J Cardiovasc Electrophysiol. 2006. 17:1068–1071.

11. Yu CM, Chan JY, Zhang Q, Omar R, Yip GW, Hussin A, Fang F, Lam KH, Chan HC, Fung JW. Biventricular pacing in patients with bradycardia and normal ejection fraction. N Engl J Med. 2009. 361:2123–2134.

12. Janousek J, Gebauer RA, Abdul-Khaliq H, Turner M, Kornyei L, Grollmuss O, Rosenthal E, Villain E, Früh A, Paul T, Blom NA, Happonen JM, Bauersfeld U, Jacobsen JR, van den Heuvel F, Delhaas T, Papagiannis J, Trigo C. Working Group for Cardiac Dysrhythmias and Electrophysiology of the Association for European Paediatric Cardiology. Cardiac resynchronization therapy in pediatric and congenital heart disease: differential effects in various anatomical and functional substrates. Heart. 2009. 95:1165–1171.
13. Chen CA, Wang SS, Chiu SN, Wu ET, Lin MT, Wang JK, Wu MH. Left ventricular reverse remodeling after successful cardiac resynchronization therapy in a 3-year-old girl with idiopathic dilated cardiomyopathy. Int J Cardiol. 2007. 117:e7–e9.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download