Abstract
It is reported that a conversion from mycophenolate mofetil (MMF) to enteric-coated mycophenolate sodium (EC-MPS) relieves gastrointestinal (GI) symptom burden and improves health-related quality of life (HRQoL). However, it is unclear whether renal transplant recipients using tacrolimus receive the same benefit from the conversion. In this prospective, multi-center, open-label trial, patients were categorized into two groups by their GI symptom screening. Equimolar EC-MPS (n=175) was prescribed for patients with GI burdens; those with no complaints remained on MMF (n=83). Gastrointestinal Symptom Rating Scale (GSRS) and Gastrointestinal Quality of Life Index (GIQLI) were evaluated at baseline and after one month. Patients and physicians completed Overall Treatment Effect (OTE) at one month. EC-MPS-converted patients had worse GSRS and GIQLI scores at baseline than MMF-continued patients (all P<0.001). Significant improvements in GSRS and GIQLI scores were observed for EC-MPS-converted patients at one month, but MMF-continued patients showed worsened GSRS scores (all P<0.05). OTE scale indicated that EC-MPS patients improved in overall GI symptoms and HRQoL more than MMF patients did (P<0.001). In tacrolimus-treated renal transplant recipients with GI burdens, a conversion from MMF to EC-MPS improves GI-related symptoms and HRQoL.
Mycophenolate mofetil (MMF) is one of the most successful medications used in renal transplantation, showing reduction in acute rejection and late allograft loss (1-3). However, gastrointestinal (GI) side effects are common, and inadequate mycophenolic acid (MPA) exposure can limit its clinical benefits (4). Enteric-coated mycophenolate sodium (EC-MPS) has been developed to improve GI tolerability of MPA treatment. Conversion from MMF to EC-MPS can be safely undertaken without compromising efficacy de novo and maintenance of renal transplant recipients (5, 6).
GI symptoms and subsequently impaired quality of life is prevalent among renal transplant recipients (7). It was previously demonstrated that a conversion from MMF to EC-MPS improved GI-related symptoms and health-related quality of life (HRQoL) in a study using patient-reported outcomes measures (8, 9). However, such benefits obtained from converting to EC-MPS is not easily applicable in patients receiving tacrolimus; since MPA therapy provides pharmacokinetic interaction with tacrolimus, MPA exposure would be augmented in combination with tacrolimus (10-12). Furthermore, tacrolimus has its own inherent GI side effects (7, 13). Thus, patients who receive MPA therapy and tacrolimus simultaneously can experience more severe GI disorders and impaired HRQoL.
Based on these findings, the present study was undertaken with renal transplant recipients receiving tacrolimus to investigate the impact of conversion from MMF to EC-MPS. The aim of this study was to determine whether patients with GI complaints can experience improvement on symptom severity and GI-specific HRQoL after converting to EC-MPS. The impact of EC-MPS conversion on general HRQoL was also investigated; the result was then compared with that of patients without GI complaints who remained under MMF.
study consisted of multicenter, longitudinal, open-label, and prospective trials in adult renal transplant patients receiving MMF (CellCept, Roche) in combination with tacrolimus. Patients were recruited at seven transplant centers (Seoul St. Mary's hospital, Samsung Seoul Medical Center, Seoul National University hospital, Bong Seng Memorial hospital, Busan Paik hospital, Kyungpook National University hospital and Ajou University Medical Center) in Korea. All patients completed a simple questionnaire that classified them into EC-MPS (Myfortic, Novartis Pharmaceuticals Corporation)-converted or MMF-continued group, and patient-reported outcomes measures were evaluated at baseline and one month (Fig. 1).
Patients aged 18-65 yr were eligible to enter the study if they had received a renal transplant at least three months prior to the research and had been receiving a combination of MMF and tacrolimus with or without corticosteroids for at least two weeks at the time of study entry. Patients were ineligible to enter the study if they were experiencing GI symptoms that were not known or assumed to be caused by MMF, had experienced an episode of acute rejection, or were undergoing acute medical illness within two weeks prior to study entry. Patients were also excluded if they satisfied following criteria: multiorgan recipients; positive T-cell crossmatch; ABO incompatibility against donor; serum creatinine >2.0 mg/dL at screening or baseline; leukopenia (<2,500 cells/µL) and/or absolute neutrophil count of <1,500 cells/µL; thrombocytopenia (<75,000 cells/µL); hepatitis B or C virus infection.
At study entry, all patients completed a simple questionnaire for whether they had experienced GI symptoms during previous three months. This questionnaire has six subscales (indigestion, bloating, upper abdominal pain, nausea, vomiting and diarrhea) that have a six-point graded Likert-type scale, where 0 represents no discomfort and 5 represents continual discomfort. For patients with total subscale scores above or equal to 2, prescriptions were converted from MMF to EC-MPS (EC-MPS-converted group). Patients with total subscale scores below 2 remained on MMF medication (MMF continued group).
Patients in EC-MPS-converted group were converted from MMF to an equimolar dose of EC-MPS at baseline; EC-MPS doses of 360 mg, 720 mg, 1,080 mg, and 1,440 mg corresponded to MMF 500 mg, 1,000 mg, 1,500 mg, and 2,000 mg, respectively. Dose reduction or temporary interruption of EC-MPS or MMF was permitted in cases of leukopenia (<4,500 cells/µL), neutropenia (<1,500 cells/µL), thrombocytopenia (<75,000 cells/µL), or in response to moderate or severe adverse events. Discontinuation of EC-MPs or MMF was considered if it was interrupted for longer than 10 days. Tacrolimus with or without corticosteroids was administered according to the participating center protocols.
Two self-administered questionnaires were used (14). The Gastrointestinal Symptom Rating Scale (GSRS) is a 15-item instrument designed to assess common GI symptoms. It has five subscales (reflux, diarrhea, constipation, indigestion, and abdominal pain) with subscale scores ranging from 1 (no discomfort) to 7 (severe discomfort). Higher scores represent higher symptom burden. The Gastrointestinal Quality of Life Index (GIQLI) is a 36-item GI-specific HRQoL instrument designed to assess the quality of life in patients with GI disorders. It has five subscales (GI symptoms, social function, physical function, emotional status, and use of medical treatment) and a total of 36 items, with a total score ranging from 0 to 144. Higher scores mean better GI specific HRQoL. Patients completed GSRS and GIQLI questionnaires at each study visit.
The effect of the treatment in terms of HRQoL changes was investigated at end of one month, using the Overall Treatment Effect (OTE) scale. The first question asks whether general HRQoL has improved, remained the same, or deteriorated. Respondents who indicated improvement or deterioration were further asked to identify the extent of the change on the 7-point scale, with 1 being almost the same and 7 being great deal better or worse. In addition, physicians also completed the OTE questionnaire for symptom assessment at the end of one month.
The primary efficacy variable was the change in overall GSRS score from the baseline within one month in EC-MPS-converted patients. Secondary efficacy variables included the change in GIQLI from the baseline within one month, the change from baseline in GSRS and GIQLI subscale scores, and the OTE scores by patients and physicians. Safety variables included the occurrence of adverse events, serious or severe adverse events, medication stop, acute rejection, graft loss, and death.
Efficacy analyses were performed on per-protocol population, which is defined as all patients who completed the study with no protocol violations. Safety analyses were performed on ITT/safety population, which consisted of all patients who received at least one dose of EC-MPS or MMF. Patient-reported outcomes results are reported as mean±SD. Baseline, one month scores, and changed scores in GSRS and GIQLI overall and all subscales was compared using Student t test between EC-MPS converted and MMF continued groups. Changes in GSRS and GIQLI overall and subscale scores within groups were tested using a paired t test. Fisher's exact test or chi-square test was used for categorical variables. All statistical tests used were two-sided with a significance level of 0.05.
A total of 276 patients who enrolled (188 in EC-MPS-converted group and 88 in MMF-continued group) comprised the ITT/safety population. Among them, thirteen patients (6.9%) in EC-MPS-converted group and five patients (5.7%) in MMF-continued group discontinued prematurely (11, administrative problems; 3, adverse events; 3, subject withdrew consent; 1, death). The per-protocol population consisted of 258 patients--175 in EC-MPS-converted group and 83 in MMF-continued group.
Table 1 describes the baseline patient demographics. Patients in EC-MPS-converted group had a greater prevalence of younger, taller and female patients. The daily MMF doses before conversion were not different between two groups. When patients were grouped based on simple questionnaire scores, EC-MPS-converted group had a significantly higher mean score than MMF continued group (6.85±4.02 vs 0.40±0.49; P<0.001; Fig. 2). All subscale scores for the simple questionnaire were also different, and most pronounced distinctions in the symptoms were bloating and upper abdominal pain.
The overall GSRS score at the baseline was 2.46±0.81 in EC-MPS-converted group and 1.54±0.51 in MMF continued group (Fig. 3). This difference indicates a significantly worse symptom burden in EC-MPS-converted group (P<0.001). EC-MPS-converted group also had significantly higher scores on all GSRS subscales and lower scores on GIQLI overall and subscale scores than MMF continued group (all P<0.001). The overall GIQLI score was 2.94±0.48 for EC-MPS-converted group and 3.35±0.34 for MMF continued group.
The overall GSRS score after one month was 2.11±0.74 in EC-MPS-converted group, which was significantly lower than the baseline score (P<0.001). All GSRS subscale scores except constipation were improved in EC-MPS-converted group (P=0.426 for constipation and all P<0.05 for other subscales). On the other hand, overall GSRS score in MMF continued group increased after one month (1.54±0.51 at baseline vs 1.71±0.64 at one month; P=0.002), and all subscales scores except diarrhea deteriorated (P=0.271 for diarrhea and all P<0.05 for other subscales). When the changed GSRS scores between two groups were compared, the overall GSRS score was significantly reduced in EC-MPS-converted group (Fig. 4A), and the difference remained significant even after the scores were adjusted for age, sex, height and investigation centers (P<0.001). The changed scores in all GSRS subscales were also different between two groups, with the greatest change reported in abdominal pain subscale (all P<0.05).
Significant improvement after one month was observed for GIQLI overall and all subscale scores in EC-MPS-converted group (all P<0.05). The overall GIQLI score after one month was 3.11±0.41, with the greatest change reported in GI symptom subscale (P<0.001). In MMF continued group, overall GIQLI (3.35±0.34 at baseline vs 3.30±0.39 at one month; P=0.085) and some subscale scores after one month remained stable, or even decreased in GI symptoms and medical treatment subscale (P=0.031 and P=0.012, respectively). In comparison to MMF continued group, EC-MPS-converted group had significantly different changes in GIQLI overall and subscale scores, even after adjusting the scores for demographic and clinical characteristics (P<0.05) (Fig. 4B).
Patient-rated OTE after one month showed that 70 patients (40.0%) in EC-MPS-converted group made an improvement in their HRQoL in comparison to the baseline, while only seven patients (8.4%) in MMF-continued group reported such an improvement (Fig. 5). For physician-rated OTE, GI symptom improved, remaining unchanged, or worsened in 79 patients (45.1%), 82 patients (46.1%) and 14 patients (8.9%) in EC-MPS-converted group, respectively. In MMF continued group, two (2.4%), 73 (88.0%), and eight patients (9.6%) displayed such a pattern. Thus, distributions for patient and physician-rated OTE were significantly different between two groups (all P<0.001).
A total of 38 GI adverse events were developed, which indicated no significant difference between EC-MPS-converted and MMF-continued groups (14.4% vs 12.5%; P<0.676). Of these events, the percentage of severe adverse events (2.1% vs 0%; P=0.310), adverse events related to study drug (10.1% vs 5.7%; P=0.260), and events that required further treatment (9.0% vs 8.0%; P=0.765) were not significantly different between EC-MPS and MMF converted group. Diarrhea was the most frequently-occurring GI complaint with nine incidents reported (4.8%) in EC-MPS-converted group and one event (1.1%) in MMF continued group (P=0.177). The five patients (2.7%) who discontinued medication for GI adverse events were present in only EC-MPS-converted group, but their percentage in comparison to the total number of participants in EC-MPS-converted group was not significantly different from that of MMF continued group (P=0.181).
Seventy-six non-GI adverse events were present in study population. The frequency of non-GI adverse events in EC-MPS-converted group was significantly higher compared to that of MMF continued group (33.5% vs 14.8%; P=0.001). Common cold was the most common non-GI adverse event, reported in seventeen times (9.0%) in EC-EMP-converted group and two (2.3%) times in MMF continued group (P=0.042). The incidence of non-GI adverse events with a suspected relation to study drug was not significantly different between two test groups (EC-MPS 3.2% vs MMF 1.1%; P=0.437). In EC-MPS-converted group, four patients (all, infection) experienced non-GI serious adverse events. Four patients (2, infection; 2, nervous system disorders) discontinued EC-MPS permanently due to non-GI adverse events. In MMF continued group, no patient experienced serious adverse event or discontinued the prescription. In that regard, the incidences of serious adverse events and discontinuance of medication were not statistically different between two groups (all P=0.310). Two patients died from cerebral aspergillosis and pneumonia in EC-MPS converted group, but no episodes of acute rejection and graft loss were reported in patients of both groups.
With a increasing success in renal grafts and a decrease in patient mortality, there is a growing need to optimize immunosuppressants for improving HRQoL in renal transplant patients. This study demonstrates that a conversion from MMF to EC-MPS was effective in improving GI symptom and HRQoL in renal transplant recipients receiving tacrolimus. Significant improvements were consistently observed for GSRS and GIQLI scales, even though all of EC-MPS-converted patients already expressed GI complaints in a pre-conversion survey. The greatest improvement after converting to EC-MPS was observed in the GSRS abdominal pain subscale, and forty percent of patients reported an improvement in OTE scales in terms of HRQoL after converting from MMF to EC-MPS.
In previous trials, converting patients from MMF to EC-MPS significantly improved GI symptom burden, patient functioning, and well-being in renal transplant recipients (8, 9, 15-17). However, those trials did not include MMF control group and the study population was screened by physicians only. GI symptoms tend to be underestimated by physicians, and patients do not disclose such information voluntarily. Thus, physician judgments provided a limited amount of information for evaluating patient reported outcomes (18-20). In the present study, all patients were screened by a simple questionnaire method, and its score was used as a criteria for patient grouping. Consequently, GSRS scores at the baseline were significantly different between the two groups. Therefore, the present study is a more effective method of evaluating patient-reported outcomes in terms of grouping patients on the basis of their own reported symptoms.
The GSRS score in EC-MPS-converted group was significantly improved, but it was worsened in MMF continued group. In addition, non-significant trend towards deteriorated GIQLI score was observed for MMF continued group. These findings suggest that patients who received a simultaneous treatment of MMF and tacrolimus can experience newly developed GI symptoms or a lower HRQoL despite the little presence of GI symptoms at the baseline. Thus, further investigation is needed to determine whether EC-MPS conversion prevented GI symptoms and to improved HRQoL in MMF-treated renal transplant patients who did not suffer from GI disorder.
Diarrhea is a frequent adverse event observed during MMF treatment in renal transplant recipients. Conversion to EC-MPS conversion provided a great benefit on diarrhea (8, 9, 21). However, diarrhea subscale did not show the greatest improvement after EC-MPS conversion in this study-its beneficial effect was relatively small in contrast to previous reports. Although various parameters were related to this result, tacrolimus could be one of the most significant influences. Because tacrolimus is independently associated with diarrhea in renal transplant recipients, its GI adverse effect could offset the improvement of the symptom in EC-MPS conversion (7). In addition, a little change in constipation subscale of EC-MPS-converted group is attributed to tacrolimus, because it is also closely associated with constipation. Therefore, it is recommended that tacrolimus-related GI side effects should be considered as one of the causes in patients' persistent GI symptom after EC-MPS conversion.
After comparing the overall baseline GIQLI score between EC-MPS-converted and MMF-continued groups, it was revealed that patients with GI complaints had 12% lower overall GIQLI score than those without. As expected, a reduced GI symptom burden after EC-MPS conversion significantly increased GI-specific HRQoL. It is noteworthy that EC-MPS conversion significantly improved the overall functioning and well-being of patients, as indicated by patient-rated OTE. The impact of converting to EC-MPS on general HRQoL was also supported by MMF-continued patients, whose patient-rated OTE remained unchanged. These findings suggest that GI related symptom and HRQoL are closely associated with general HRQoL in renal transplant recipients, and that EC-MPS conversion could be one of the methods for improving the overall HRQoL.
The overall incidence of non-GI adverse events in EC-MPS-converted group was significantly higher than that of MMF continued group. However, the incidence of non-GI adverse events with a suspected relation to study drug, serious adverse events, and discontinuation of study drug were similar between both groups. In addition, baseline characteristics in EC-MPS-converted group showed a greater prevalence of younger and female patients, and it might be associated with increment of non-GI adverse events. Therefore, greater non-GI adverse events of EC-MPS-converted group in this trial could not be the barrier for EC-MPS conversion.
In the present study, placebo effect can not be completely ruled out. Because neither investigators nor patients were blinded, EC-MPS conversion might have implied to the patients that GI-side effects would be alleviated. However, the significant improvements of GSRS and GIQLI score scores were observed after EC-MPS conversion, and OTE rated by patients and physicians showed consistent results. These findings suggest that observed improvements cannot be explained solely by the placebo effect.
In conclusion, a conversion from MMF to EC-MPS improves GI-related symptom and HRQoL in tacrolimus-treated renal transplant recipients with GI burdens. The efficacy and safety of EC-MPS conversion helps to optimize patient functioning and well-being. Ultimately, these improvements translate into an increased medication compliance and adherence, which may lead to further improvements in graft survival rates.
Figures and Tables
Fig. 1
Schematic diagram of study design and conduct.
EC-MPS, enteric-coated mycophenolate sodium; MMF, mycophenolate mofetil; GSRS, Gastrointestinal Symptom Rating Scale; GIQLI, Gastrointestinal Quality of Life Index.
Fig. 2
Simple questionnaire screening for GI symptoms. *denotes P values <0.001. EC-MPS, enteric-coated mycophenolate sodium; MMF, mycophenolate mofetil.

Fig. 3
Patient-reported outcomes at baseline in EC-MPS converted and MMF continued group. (A) GSRS subscale scores (B) GIQLI subscale scores.
*denotes P values <0.001. Note the significantly impaired GSRS and GIQLI all subscale scores in EC-MPS converted group.
EC-MPS, enteric-coated mycophenolate sodium; MMF, mycophenolate mofetil; GSRS, Gastrointestinal Symptom Rating Scale; GIQLI, Gastrointestinal Quality of Life Index.
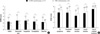
Fig. 4
Changes in patient-reported outcomes after one month in EC-MPS converted and MMF continued group. (A) changes in GSRS subscale (B) changes in GIQLI subscale scores.
*denotes P values <0.05. EC-MPS converted group significantly improved in GSRS and GIQLI all subscale scores compared to MMF continued group.
EC-MPS, enteric-coated mycophenolate sodium; MMF, mycophenolate mofetil; GSRS, Gastrointestinal Symptom Rating Scale; GIQLI, Gastrointestinal Quality of Life Index.

Fig. 5
Overall Treatment Effect (OTE) rating for (A) patient-rated general health-related quality of life (HRQoL) and (B) physician-rated GI symptoms after conversion to EC-MPS compared to baseline. EC-MPS converted group significantly improved in patient- and physician-rated OTE compared to MMF continued group (all P<0.001).
EC-MPS, enteric-coated mycophenolate sodium; MMF, mycophenolate mofetil.

ACKNOWLEDGMENTS
The authors would like to acknowledge the collaboration and commitment of all the local investigators and their staff, without whom the present study would not have been possible.
References
1. The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation. 1996. 61:1029–1037.
2. Meier-Kriesche HU, Steffen BJ, Hochberg AM, Gordon RD, Liebman MN, Morris JA, Kaplan B. Long-term use of mycophenolate mofetil is associated with a reduction in the incidence and risk of late rejection. Am J Transplant. 2003. 3:68–73.

3. Kang NR, Lee JE, Huh W, Kim SJ, Kim YG, Kim DJ, Oh HY. Minimal proteinuria one year after transplant is a risk factor for graft survival in kidney transplantation. J Korean Med Sci. 2009. 24:Suppl 1. S129–S134.

4. Bunnapradist S, Lentine KL, Burroughs TE, Pinsky BW, Hardinger KL, Brennan DC, Schnitzler MA. Mycophenolate mofetil dose reductions and discontinuations after gastrointestinal complications are associated with renal transplant graft failure. Transplantation. 2006. 82:102–107.

5. Salvadori M, Holzer H, de Mattos A, Sollinger H, Arns W, Oppenheimer F, Maca J, Hall M. ERL B301 Study Groups. Enteric-coated mycophenolate sodium is therapeutically equivalent to mycophenolate mofetil in de novo renal transplant patients. Am J Transplant. 2004. 4:231–236.
6. Budde K, Curtis J, Knoll G, Chan L, Neumayer HH, Seifu Y, Hall M. ERL B301 Study Groups. Enteric-coated mycophenolate sodium can be safely administered in maintenance renal transplant recipients: results of a 1-year study. Am J Transplant. 2004. 4:237–243.
7. Ekberg H, Kyllönen L, Madsen S, Grave G, Solbu D, Holdaas H. Increased prevalence of gastrointestinal symptoms associated with impaired quality of life in renal transplant recipients. Transplantation. 2007. 83:282–289.

8. Bolin P, Tanriover B, Zibari GB, Lynn ML, Pirsch JD, Chan L, Cooper M, Langone AJ, Tomlanovich SJ. Improvement in 3-month patient-reported gastrointestinal symptoms after conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in renal transplant patients. Transplantation. 2007. 84:1443–1451.

9. Chan L, Mulgaonkar S, Walker R, Arns W, Ambühl P, Schiavelli R. Patient-reported gastrointestinal symptom burden and health-related quality of life following conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium. Transplantation. 2006. 81:1290–1297.

10. Kaplan B, Meier-Kriesche HU, Minnick P, Bastien MC, Sechaud R, Yeh CM, Balez S, Picard F, Schmouder R. Randomized calcineurin inhibitor cross over study to measure the pharmacokinetics of co-administered enteric-coated mycophenolate sodium. Clin Transplant. 2005. 19:551–558.

11. Zucker K, Rosen A, Tsaroucha A, de Faria L, Roth D, Ciancio G, Esquenazi V, Burke G, Tzakis A, Miller J. Unexpected augmentation of mycophenolic acid pharmacokinetics in renal transplant patients receiving tacrolimus and mycophenolate mofetil in combination therapy, and analogous in vitro findings. Transpl Immunol. 1997. 5:225–232.

12. Filler G, Zimmering M, Mai I. Pharmacokinetics of mycophenolate mofetil are influenced by concomitant immunosuppression. Pediatr Nephrol. 2000. 14:100–104.

13. Pirsch JD, Miller J, Deierhoi MH, Vincenti F, Filo RS. FK506 Kidney Transplant Study Group. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. Transplantation. 1997. 63:977–983.
14. Kleinman L, Faull R, Walker R, Ramesh Prasad GV, Ambuehl P, Bahner U. Gastrointestinal-specific patient-reported outcome instruments differentiate between renal transplant patients with or without GI complications. Transplant Proc. 2005. 37:846–849.
15. Cofan F, Rosich E, Arias M, Torregrosa V, Oppenheimer F, Campistol JM. Quality of life in renal transplant recipients following conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium. Transplant Proc. 2007. 39:2179–2181.

16. Darji P, Vijayaraghavan R, Thiagarajan CM, Sharma RK, Subbarao B, Pishardy R, Dakshinamurthy KV, Vijaykumar R, Abraham G, Bhaskar S, Agarwal L, Shah B, Abraham A, John M, Sampathkumar K, Das T, Umesh L, Sundar S, Ballal H, Jasuja S, Saxena S, Saha TK. Conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in renal transplant recipients with gastrointestinal tract disorders. Transplant Proc. 2008. 40:2262–2267.

17. Shehata M, Bhandari S, Venkat-Raman G, Moore R, D'Souza R, Riad H, Bakran A, Baker R, Needham C, Andrews C. Effect of conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium on maximum tolerated dose and gastrointestinal symptoms following kidney transplantation. Transpl Int. 2009. 22:821–830.

18. McColl E, Junghard O, Wiklund I, Revicki DA. Assessing symptoms in gastroesophageal reflux disease: how well do clinicians' assessments agree with those of their patients? Am J Gastroenterol. 2005. 100:11–18.

19. Fallone CA, Guyatt GH, Armstrong D, Wiklund I, Degl'Innocenti A, Heels-Ansdell D, Barkun AN, Chiba N, Zanten SJ, El-Dika S, Austin P, Tanser L, Schünemann HJ. Do physicians correctly assess patient symptom severity in gastro-oesophageal reflux disease? Aliment Pharmacol Ther. 2004. 20:1161–1169.

20. Jones RH, Hungin AP, Phillips J, Mills JG. Gastro-oesophageal reflux disease in primary care in Europe: clinical presentation and endoscopic findings. Eur J Gen Pract. 1995. 1:149–154.

21. Behrend M. Adverse gastrointestinal effects of mycophenolate mofetil: aetiology, incidence and management. Drug Saf. 2001. 24:645–663.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download