Abstract
The purpose of this study was to explore the role of epithelial-mesenchymal transition in the pathogenesis of hepatolithiasis. Thirty-one patients with primary hepatolithiasis were enrolled in this study. Expressions of E-cadherin, α-catenin, α-SMA, vimentin, S100A4, TGF-β1 and P-smad2/3 in hepatolithiasis bile duct epithelial cells were examined by immunohistochemistry staining. The results showed that the expressions of the epithelial markers E-cadherin and α-catenin were frequently lost in hepatolithiasis (32.3% and 25.9% of cases, respectively), while the mesenchymal markers vimentin, α-SMA and S100A4 were found to be present in hepatolithiasis (35.5%, 29.0%, and 32.3% of cases, respectively). The increased mesenchymal marker expression was correlated with decreased epithelial marker expression. The expressions of TGF-β1 and P-smad2/3 in hepatolithiasis were correlated with the expression of S100A4. These data indicate that TGF-β1-mediated epithelial-mesenchymal transition might be involved in the formation of hepatolithiasis.
Primary hepatolithiasis (HL), a condition marked by the presence of calculus in intrahepatic ducts, is a common disease in China, especially prevalent in the Eastern and Southern regions of China. Although HL is a benign disease, it is intractable and usually requires operative interventions due to frequent stone recurrences (1). Most importantly, this disease is associated with biliary cirrhosis, and even cholangiocarcinoma. Clinical and experimental studies demonstrated that hepatolithiasis is associated with chronic inflammation of bile ducts, biliary fibrosis, biliary stricture, and bacterial infections (2, 3). However, the cellular and molecular pathogenesis of hepatolithiasis remains elusive. Many previous studies have focused on the formation of stones, while little attention has been paid to the role of bile duct epithelial cells (BEC).
Epithelial-mesenchymal transition (EMT) is, by definition, a process whereby epithelial cells are converted to migratory mesenchymal cells. This process is characterized by a loss of cell polarity and acquired expression of mesenchymal components (4). EMT results in a dramatic remodeling of the cytoskeleton, which includes reduced expression of cell adhesion molecules (i.e., E-cadherin) and the transition of keratin-based cytoskeleton into vimentin-based cytoskeleton, thus leading to changes in cell morphology. It has been reported that EMT contributes to both renal fibrogenesis and the fibrogenetic processes of hepatocirrhosis (5, 6).
It has been shown that pleiotropic cytokines, members of the transforming growth factor-β (TGF-β) family, are involved in multiple physiological and pathological processes, including cellular proliferation, adhesion, apoptosis, differentiation and immunoregulatory activities. TGF-β1 activates Smad signaling via its two cell surface receptors-TGF-β type I receptor (TβRI) and TGF-β type II receptor (TβRII), leading to Smad-mediated transcriptional regulation of target genes (7, 8). Recently, Rygiel et al. reported that bile duct epithelial cells underwent EMT during chronic liver diseases, which was associated with TGF-β1 (9).
Based on the observations above, we supposed that mesenchymal transition of BECs also play a role in the development of primary hepatolithiasis. Therefore, this study aimed at exploring whether intrahepatic biliary epithelial cells undergo EMT in hepatolithiasis, and the possible role of TGF-β1-Smad2/3 signal pathway in EMT in hepatolithiasis.
The hepatolithiasis tissue samples were obtained from the Institute of Hepatobiliary Surgery of Southwest Hospital, the Third Military Medical University, with the approval of the Ethics Committee of the Third Military Medical University (IRB approval ID: KY200803). Written informed consent was obtained from all patients.
During March 2008 and December 2008, a total of 31 surgically resected hepatolithiasis specimens from primary hepatolithiasis were collected at the two ends of the stones (9 cases of male, 22 cases of female, average age 47.6±11.4 yr). Of the 31 cases of HL, 25 patients had a history of cholangitis. There were 16 cases of left hepatic duct stones, including five cases accompanied with choledocholithiasis and one case accompanied with cholecystolithiasis. There were 8 cases of right hepatic duct stones, three cases were accompanied with choledocholithiasis. There were 7 cases of combined left and right HL, four cases were accompanied with comorbid choledocholithiasis and three cases were accompanied with comorbid cholecystolithiasis. Normal surrounding tissues (paraffin embedded) of 18 cases of cavernous hemangioma of the liver were used as the control.
All patients with hepatolithiasis were pathologically diagnosed and graded into three grades according to the distribution of stones and pathological changes as described previously (10). Briefly, Grade I (localized) was characterized by stones that were confined to the hepatic segments or hepatic bile ducts, with liver involvement and few pathological changes in bile ducts. Grade II (regional) was characterized by a regional distribution of stones in the intrahepatic bile ducts permeating into one or several hepatic segments, combined with biliary duct stenosis and involvement of hepatic atrophy. Grade III (diffuse type) was characterized with or without obvious hepatic parenchymal atrophy and fibrosis, combined with stenosis of the main hepatic ducts in diseased regions or as having extensive hepatic fibrosis and formation of secondary biliary cirrhosis and portal hypertension, combined with the serious stenosis. Cholangiocarcinoma was excluded through pathological diagnosis.
All resected specimens were fixed in 4% paraformaldehyde, embedded in paraffin and cut into 4-µm-thick sections. Before IHC staining, all sections were deparaffinized and heated in citrate buffer (10 mM/L citric acid, pH 6.0) in a microwave oven. After inactivation by exposure to 3% H2O2 for 10 min, which blocks the activity of endogenous peroxidases, the sections were incubated with blocking serum at room temperature for 10 min. IHC staining was carried out with the primary antibodies anti-α-SMA (Dako, Glostrup, Denmark), anti-E-cadherin, anti-α-catenin, anti-vimentin, anti-S100A4, anti-TGF-β1, and anti-P-smad2/3 (Santa Cruz, CA, USA). After incubation with secondary antibodies, expressions of these markers in cells were detected with diaminobenzidine (DAB; Sigma, St. Louis, MO, USA) by enhancement with a SABC kit (ZSGB-BIO, Beijing, China). Tissue sections stained without primary antibody served as a negative control. The slides were then counterstained with hematoxylin. Images were captured by Baumer digital camera with an Olympus BX51 microscopy (Olympus, Tokyo, Japan).
Brown granular staining was considered to be a positive signal for the IHC assay. On each slice, BECs of at least 10 portal areas were counted and the positive rates were calculated manually. The portal areas were chosen randomly, without considering the magnitude of bile duct. Immunostaining of E-cadherin and α-catenin was evaluated according to the method described previously (11). N-cadherin expression was considered to be normal if >90% of BECs exhibited a staining pattern similar to that seen in normal epithelial cells; sections with <90% of BECs stained were classified as having a "lost pattern". The intensity of vimentin, α-SMA and S100A4 was determined in the same way as that of N-cadherin staining (12). Briefly, the samples were divided into two groups based on the intensity of staining these mesenchymal markers in BECs: a positive group in which >20% of BECs were stained positively and a negative group in which <20% of BECs were stained positively.
Measurement data was compared with t test, and chi-squared test was performed to compare numerical data. A P value <0.05 was considered statistically significant. All calculations were performed on a personal computer using the statistical software package SPSS 13.0 (Statistical Package for the Social Sciences; SPSS, Munich, Germany).
According to the clinical information and histopathologic change, 31 cases were classified as grade I (n=10 cases), grade II (n=8 cases) and grade III (n=13 cases). All sections of HL showed different degree of infiltration of inflammatory cells in portal areas, fibrous tissue hyperplasia, biliary dilatation, and necrosis (Fig. 1).
E-cadherin and α-catenin were expressed in the plasma membrane as continuous linear staining and the cytoplasm. The E-cadherin- and α-catenin-positive BECs in HL were less than that in control group (P=0.001, and P=0.012, respectively). In HL group, the cases with less than 90% BECs expressing E-cadherin accounted for 32.3%, significantly higher than that in control group (P=0.038) (Fig. 2). Conversely, vimentin-, α-SMA- and S100A4-positive BECs in HL were significantly more than that in control group (Table 1). In HL group, the cases with more than 20% BECs expressing vimentin, α-SMA and S100A4 accounted for 35.5%, 29.0% and 32.3%, respectively, significantly higher than those in control group (P=0.036, P=0.018, and P=0.008,) (Fig. 2C-F). There was very little expression of these three mesenchymal proteins in the control group. Collectively, there was increased expression of mesenchymal markers and reduced expression of epithelial markers in HLBECs, which indicated that EMT might be involved in the development to HL.
Although the expressions of TGF-β1 and P-smad2/3 were not significantly different from that in the control tissues (38.7% vs. 16.7% and 48.4% vs. 22.2%, respectively, P>0.05), the expressions of TGF-β1 and P-smad2/3 were parallel with the expressions of the mesenchymal markers (vimentin, α-SMA and S100A4) (Fig. 3). Positive relationship between TGF-β1 or P-smad2/3 and S100A4 was observed (r=0.737, and r=0.658, respectively). These results indicate that TGF-β1 may play an important role in EMT in the development of HL.
The relationship between the expression of the epithelial and mesenchymal markers and clinical characteristics is shown in Table 2. The expression of S100A4 was associated with the clinical grading of HL. Although no statistical relationship between the other markers and clinical grading of HL was observed, there was a tendency of negative correlation between clinical severity and the expression of E-cadherin and α-catenin (P=0.075 and P=0.052), and a tendency of positive correlation between clinical severity and vimentin and α-SMA (P=0.078, P=0.085).
A number of recent studies have suggested that loss of epithelial markers and gain of mesenchymal markers defines the process of EMT (13). The statistical analysis suggested that the presence of mesenchymal markers was associated with the lost of epithelial markers in HL (Table 3), which further supports the existence of EMT.
Primary hepatolithiasis is a common and benign disease in China, and the clinical progression of this disease may result in bile duct stricture, liver parenchyma atrophy and chronic fibrosis due to repeated pyogenic cholangitis (14). Moreover, cholangiocarcinoma is found in approximately 5-10% of patients with hepatolithiasis (2). Therefore, it is essential to explore the cellular and molecular mechanisms that mediate the pathogenesis of hepatolithiasis.
EMT, first appreciated as a developmental event in early embryonic morphogenesis, is a process whereby epithelial cells lose polarity and cell-cell contacts and acquire the ability to express mesenchymal components and manifest a migratory phenotype (13, 15). Studies have revealed that the morphological transition from an epithelial to a fibroblastic appearance is accompanied by a gain of mesenchymal cell markers (fibronectin, vimentin, smooth muscle, actin, and N-cadherin) and a loss of epithelial markers (E-cadherin, and α- and β-catenin) (16). EMT-like changes are thought to be involved in many pathological processes, such as renal fibrosis (17), epithelial hyperplasia and tumor metastasis (13). Moreover, the studies suggest that EMT plays a critical role in liver and biliary diseases. Consistent with these studies, our results showed that the bile duct epithelial cells in HL underwent a lost of epithelial markers and acquirement of mesenchymal markers, suggesting the presence of EMT. Meanwhile, this study indicates that the expression of epithelial and mesenchymal markers is associated with clinical severity, which showed that local inflammatory factors may be involved in the development of EMT while leading tissue injury.
Of the many factors that regulate EMT, TGF-β1 is regarded as a key cytokine that induces EMT through Smads and Smads-independent pathways (13, 16). Treatment with TGF-β1 was sufficient to induce EMT in BECs, as demonstrated by loss of E-cadherin staining and gain of S100A4, Smad2/3, and α-SMA (18). In our study, there was no significant difference between TGF-β expressions of HL and control group. However, we find that the expression of TGF-β and Smad2/3 was associated with the expression of mesenchymal markers, especially S100A4. Our results were compatible with Rygiel's report that TGF-β1 was associated with EMT of bile duct epithelial cells during chronic liver diseases (9). However, whether EMT is caused by the combination of HL and concomitant inflammation or whether EMT induces bile duct stricture and stones needs further study. In the future, we plan to analyze the role of EMT in biliary infections through simulating infections via the administration of inflammatory cytokines to intrahepatic biliary epithelial cells (IBECs) in vitro. The interactions between BECs and infiltrating T cells will be considered as well.
In conclusion, in biliary epithelial cells from patients with primary hepatolithiasis, we found the loss of epithelial markers and the acquirement of mesenchymal markers following stimulation of the TGF-β1/smad2/3 signaling pathway. Our study indicates that TGF-β1-mediated EMT might be involved in the pathogenesis of hepatolithiasis.
Figures and Tables
Fig. 1
Histopathological assessment of HL. Histopathological assessment (hematoxylin-eosin staining) of samples from (A) control group and (B) primary hepatolithiasis, abnormalities in which include infiltrating inflammatory cells in portal areas, fibrous tissue hyperplasia, biliary dilatation, and necrosis. Magnification ×200.

Fig. 2
Immunohistochemical assessment of epithelial and mesenchymal markers. Expressions of the epithelial markers (A) E-cadherin and (B) α-catenin in primary hepatolithiasis. Some BECs lost expression of epithelial markers (arrows). Expressions of mesenchymal markers (C) Vimentin, (D) α-SMA and (E) S100A4 in the liver bile ducts in hepatolithiasis. Note the brown staining of the markers lining the plasma membrane and in the cytoplasm (arrows). In normal liver tissue, S100A4 is neganative (F). Magnification ×400.
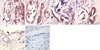
Fig. 3
Immunohistochemical assessment of TGF-β1 and P-smad2/3. (A) TGF-β1 expressed in the cytoplasm and on the plasma membrane of bile duct epithelial cells in hepatolithiasis, and (B) P-smad2/3 accumulated in the nucleus of bile duct epithelial cells in hepatolithiasis. Magnification ×400.
Table 1
Differences in the expressions of epithelial markers and mesenchymal markers in primary hepatolithiasis and control groups
References
1. Shoda J, Tanaka N, Osuga T. Hepatolithiasis-epidemiology and pathogenesis update. Front Biosci. 2003. 8.

2. Kubo S, Kinoshita H, Hirohashi K, Hamba H. Hepatolithiasis associated with cholangiocarcinoma. World J Surg. 1995. 19:637–641.

3. Nakai A, Imano M, Takeyama Y, Shiozaki H, Ohyanagi H. An immunohistochemical study of osteopontin in hepatolithiasis. J Hepatobil Pancreat Surg. 2008. 15:615–621.

4. Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002. 2:442–454.

5. Vongwiwatana A, Tasanarong A, Rayner DC, Melk A, Halloran PF. Epithelial to mesenchymal transition during late deterioration of human kidney transplants: the role of tubular cells in fibrogenesis. Am J Transplant. 2005. 5:1367–1374.

6. Robertson H, Kirby JA, Yip WW, Jones DE, Burt AD. Biliary epithelial-mesenchymal transition in posttransplantation recurrence of primary biliary cirrhosis. Hepatology. 2007. 45:977–981.

7. Schiffer M, von Gersdorff G, Bitzer M, Susztak K, Bottinger EP. Smad proteins and transforming growth factor-beta signaling. Kidney Int. 2000. 77:Suppl. S45–S52.
8. ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004. 29:265–273.
9. Rygiel KA, Robertson H, Marshall HL, Pekalski M, Zhao L, Booth TA, Jones DE, Burt AD, Kirby JA. Epithelial-mesenchymal transition contributes to portal tract fibrogenesis during human chronic liver disease. Lab Invest. 2008. 88:112–123.

10. Han DB, Dong JH, Guo GJ. Evaluation of our clinicopathologic stage for 1259 patients with intrahepatic stones. Acta Academiae Medicinae Militaris Tertiae. 2006. 28:1337–1338.
11. Asayama Y, Taguchi Ki K, Aishima Si S, Nishi H, Masuda K, Tsuneyoshi M. The mode of tumour progression in combined hepatocellular carcinoma and cholangiocarcinoma: an immunohistochemical analysis of E-cadherin, alpha-catenin and beta-catenin. Liver. 2002. 22:43–50.

12. Nakajima S, Doi R, Toyoda E, Tsuji S, Wada M, Koizumi M, Tulachan SS, Ito D, Kami K, Mori T, Kawaguchi Y, Fujimoto K, Hosotani R, Imamura M. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin Cancer Res. 2004. 10:4125–4133.

13. Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004. 118:277–279.
14. Lee TY, Chen YL, Chang HC, Chan CP, Kuo SJ. Outcomes of hepatectomy for hepatolithiasis. World J Surg. 2007. 31:479–482.

15. Inayoshi J, Ichida T, Sugitani S, Tsuboi Y, Genda T, Honma N, Asakura H. Gross appearance of hepatocellular carcinoma reflects E-cadherin expression and risk of early recurrence after surgical treatment. J Gastroenterol Hepatol. 2003. 18:673–677.

16. Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004. 117:927–939.

17. Zeisberg M, Bonner G, Maeshima Y, Colorado P, Muller GA, Strutz F, Kalluri R. Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am J Pathol. 2001. 159:1313–1321.
18. Choi HS, Savard CE, Choi JW, Kuver R, Lee SP. Paclitaxel interrupts TGF-beta1 signaling between gallbladder epithelial cells and myofibroblasts. J Surg Res. 2007. 141:183–191.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download