Abstract
Triple valve surgery is usually complex and carries a reported operative mortality of 13% and 10-yr survival of 61%. We examined surgical results based on our hospital's experience. A total of 160 consecutive patients underwent triple valve surgery from 1990 to 2006. The most common aortic and mitral valve disease was rheumatic disease (82%). The most common tricuspid valve disease was functional regurgitation (80%). Seventy-four percent of the patients were in New York Heart Association (NYHA) class III and IV. Univariate and multivariable analyses were performed to identify predictors of early and late survival. Operative mortality was 6.9% (n=11). Univariate factors associated with mortality included old age, preoperative renal failure, postoperative renal failure, pulmonary complications, and stroke. Of them, postoperative renal failure and stroke were associated with mortality on multivariable analysis. Otherwise, neither tricuspid valve replacement nor reoperation were statistically associated with late mortality. Survival at 5 and 10 yr was 87% and 84%, respectively. Ninety-two percent of the patients were in NYHA class I and II at their most recent follow-up. Ten-year freedom from prosthetic valve endocarditis was 97%; from anticoagulation-related hemorrhage, 82%; from thromboembolism, 89%; and from reoperation, 84%. Postoperative renal failure and stroke were significantly related with operative mortality. Triple valve surgery, regardless of reoperation and tricuspid valve replacement, results in acceptable long-term survival.
Several cardiac diseases such as rheumatic and degenerative valve disease, as well as endocarditis, may affect multiple valves and require double and triple valve surgery (1). Triple valve surgery (either replacement of aortic, mitral, and tricuspid valves, or combined replacement of aortic and mitral valves with tricuspid valve repair) is still a challenge for most of the surgeons due to prolonged periods of cardiopulmonary bypass and aortic cross-clamp times (1, 2). Recently, reported operative mortality after triple valve surgery is about 13% (3). Moreover, multiple valve replacement exposes the patients to added long-term prosthetic valve-related morbidities such as endocarditis, thromboembolism, anticoagulation-related hemorrhage, and paravalvular leak compared with single valve replacement (1). Finally, triple valve surgery has been associated with diminished long-term survival, with reported survival at 5 and 10 yr of 75% and 61%, respectively (3), although recent surgical results were improved compared with those of previous articles.
The current study describes the experience with 160 patients who underwent triple valve surgery (TVS) at a single center in an attempt to define early and late clinical outcomes and analyze independent predictors of adverse results.
From February 1990 to May 2006, 160 consecutive patients with multiple valve disease underwent TVS at Sejong General Hospital. Clinical, operative, and outcome variables were reviewed retrospectively. The following variables were analyzed in this study: 1) preoperative variables: gender (female), old age, preoperative New York Heart Association (NYHA) class IV, congestive heart failure, hypertension, diabetes, peripheral vascular disease, cerebrovascular disease, preoperative renal failure, endocarditis, previous heart surgery, and ejection fraction (<0.4); 2) operative variables: tricuspid valve replacement, urgency, and aortic annular enlargement; 3) postoperative variables: reexploration, heart block, postoperative pulmonary complications, renal failure, stroke, and pericardial effusion.
There were 53 men and 107 women with mean age of 60.5±19.1 yr (range, 32-75 yr). The majority of patients were in NYHA functional class III or IV. Thirty two patients (20%) had undergone previous cardiac surgery. One hundred fifty-four patients (96%) were operated on electively, and 6 patients (4%) were operated on urgently. The complete clinical profile of the patients is summarized in Table 1. Choice of the prosthesis was determined on the basis of the patient's age, special request, and comorbid factors. In the aortic position, 137 mechanical and 20 bioprosthetic valves were implanted. The majority of mechanical prostheses included the St. Jude (St. Jude Medical Inc, Minneapolis, MN, U.S.A.) (n=66), Carbomedics (Carbomedics Inc, Austin, TX, U.S.A.) (n=24), and Omniscience (Medical CV Inc, Inner Grove Heights, MN, U.S.A.) (n=19) valves. Besides, Sorin (Sorin Biomedical, Saluggia, Italy) (n=9), Edwards Mira (Edwards Lifesciences, Irvine, CA, U.S.A.) (n=9), and other mechanical valve (n=10) were used. The type of bioprosthesis was the Carpentier-Edward pericardial valve (Edwards Lifesciences) (n=16) and Hancock II porcine valve (Medtronics Inc, Minneapolis, MN, U.S.A.) (n=4). Similarly, 137 mechanical and 20 bioprosthetic valves were implanted in the mitral position, including the St. Jude (n=61), Carbomedics (n=24), Omniscience (n=20), Mira (n=11), Sorin (n=10), and other mechanical valve (n=11). The type of bioprosthesis was the Carpentier-Edward pericardial valve (Edwards Lifesciences) (n=14) and Hancock II porcine valve (Medtronics Inc.) (n=6). Only 12 valves were implanted in the tricuspid valve position, and they included 5 mechanical and 7 bioprosthetic valves: St. Jude (n=3), Carbomedics (n=1), Edwards Mira (Edwards Lifesciences) (n=1), Hancock II (n=5), and Carpentier-Edward pericardial valve (n=2).
The most common aortic valve and mitral valve disorder was rheumatic valve disease, followed by prosthetic valve dysfunction. In contrast, the most common tricuspid valve disease was functional regurgitation secondary to left heart disease, followed by rheumatic valve disease. The complete list of valve disease presentation and pathologic diagnosis is presented in Table 2.
Intraoperative transesophageal echocardiography was performed in all patients to assess the extent and severity of valve disease and the quality of the replacement or repair postoperatively. All procedures were done through a median sternotomy. Valve operations were performed under the usual methods of cardiopulmonary bypass with a single aortic cross-clamp technique. Myocardial protection strategies usually included antegrade cardioplegia introduced directly into the coronary ostia and retrograde cardioplegia through a coronary sinus cannula. Moderate hypothermia was used, and the core temperature was allowed to drift to 28℃ to 32℃. Improved protection of the ventricles was provided by topical hypothermia with saline ice slush. A Biomedicus biopump (Medtronic) has been used in all patients since 1992. Distal coronary anastomoses were performed first, followed by the valve procedures. After excision of the aortic valve, the mitral valve was approached, usually through the interatrial groove, and was assessed for possible repair versus replacement. If mitral annular reconstruction was needed in the case of extensive calcification, it was performed with the utilization of glutaraldehyde-fixed bovine pericardium before implantation of the prosthesis. After the mitral valve procedure, the aortic valve was replaced (or infrequently repaired). Then tricuspid valve repair was performed with either a modified De Vega technique or Kay technique or with an annuloplasty ring. Less frequently, the tricuspid valve was replaced. Finally the proximal coronary anastomoses were performed. The operative data are listed in Table 3.
In the postoperative period, anticoagulation with warfarin was initiated for all patients if there was no evidence of active bleeding. Target international normalized ratio (INR) was 2.5-3.0 in all patients. When triple bioprosthesis were implanted, anticoagulation was just recommended in the first 6 months postoperatively for patients without atrial fibrillation.
Late outcomes were determined from clinical records when available or from written correspondence with patients' physicians and direct patient contact with telephone interviews when necessary. All follow-up data were collected by our research personnel and 7 patients were lost to follow-up. The mean and median follow-up was 5.7±5.2 and 6.3 yr, ranged from 1 month to 16 yr. Echocardiographic data at latest follow-up were collected by contacting referring cardiologists.
Statistical analysis was performed with SPSS software 10.0 (SPSS Inc, Chicago, IL, U.S.A.). Descriptive statistics are reported as mean±standard deviation for continuous variables and as frequencies and percentages for categorical variables. Unrelated two-group comparisons were done with unpaired, two-tailed Student's t tests for continuous variables and chi-square or Fisher's exact test for categorical data. Predictors of perioperative mortality were identified using univariable and multivariable logistic regression analysis. Long-term survival and freedom from morbid events were estimated using the Kaplan-Meier method. Cox regression was used to determine the independent predictors of late outcomes. The appropriateness of variable transformations was determined by means of univariate analysis. Variables with a univariate P value of less than 0.05 or those with known biologic significance, but failing to meet this critical chi-square level, were submitted to multivariable models.
The hospital mortality was 6.9% (11 patients). Five patients died due to multiorgan failure and sepsis, three due to postoperative stroke, two due to low cardiac output, and one due to sudden cardiac arrest. In the postoperative period, the incidence of complications was 28.8% (n=46 patients, in some patients there were more than one complication). Twelve patients (7.5%) required reexploration of the mediastinum for bleeding, and 11 (6.9%) required placement of a permanent pacemaker for heart block. Other morbidities included stroke in 10 patients (6.3%), renal insuffiency in 8 patients (5%, Cr ≥2.0 mg/dL), tamponade in 6 patients (3.8%), pulmonary complications (included pneumonia, atelectasis, and pleural effusions) in 5 patients (3.1%), sternal infection in 5 patients (3.1%), and sepsis in 2 patients (1.3%). Univariate analysis revealed the following variables to be associated with increased operative mortality: old age (P=0.003), preoperative renal failure (P=0.004), postoperative pulmonary complications (P=0.038), postoperative renal failure (P<0.001), and postoperative stroke (P<0.001). Postoperative renal failure (P<0.001, OR=44.6, 95% CI 6.3-316.1) and postoperative stroke (P=0.01, OR=13.6, 95% CI 1.85-100.71) remained significant on multivariable analysis (Table 4).
During the follow-up period, an additional 12 patients died (8.1%), with 10 of them of cardiac origin: congestive heart failure (n=3), arrhythmia (n=3), prosthetic valve endocarditis (It was occurred during follow-up, n=2), acute myocardial infarction (n=1), and anticoagulation related hemorrhage (n=1). The other 2 deaths were due to noncardiac causes.
Univariate and multivariate analysis revealed only prosthetic valve endocarditis as significant (P=0.017, 0.011 respectively, OR=27.60 95% CI 2.16-352.21). Otherwise, neither tricuspid valve replacement nor reoperation were statistically associated with late mortality (Table 5).
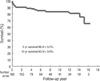
Five-year and 10-yr survival was 86.9±3.0% and 83.9±3.6%, respectively, as illustrated in Fig. 1. Ten-year freedom from prosthetic valve endocarditis was 96.6±2.0% (Fig. 2). Ten-year freedom from thromboembolism was 89.3±3.4%, whereas 10-yr freedom from anticoagulation related hemorrhage was 82.4±4.3% (Fig. 2). Ten-year freedom from other valve-related morbidities, such as structural valve deterioration, valve thrombosis, and paravalvular leakage was 88.7±3.9%, 96.0±2.4%, and 97.8±1.5%, respectively.
Twenty patients required late reoperation. Ten-year freedom from reoperation was 84.3±4.3% (Fig. 2). The causes for reoperation were as follows: pannus formation (n=9), paravalvular leakage of mitral valve (n=4), mitral valve thrombosis (n=2), failure of tricuspid valve repair (n=2), failure of aortic valve and/or mitral valve repair (n=2), prosthetic valve dysfunction after double valve replacement (n=1), acute thrombosis of aortic valve (n=1), and prosthetic valve endocarditis (n=1).
On the latest follow-up, 130 patients were alive. Among them, 17 (13.1%) were in NYHA class I, 102 (78.5%) in class II, and 11 (8.5%) in class III. All but three of the patients had normally functioning prosthetic valves at the latest echocardiographic study. Three patients are thought to be reoperated in the future: structural valve deterioration (n=2), and prosthetic valve endocarditis (n=1).
Combined surgery for aortic, mitral, and tricuspid valves (TVS) and reoperative TVS remains a formidable challenge. Despite substantial improvements in myocardial protection and CPB techniques, TVS is still a challenge for more advanced valvular disease and complexity of the operation. In addition, outcomes of TVS are often complicated by different underlying pathophysiologic conditions, associated cardiovascular diseases, and concomitant operative procedures.
The hospital mortality of 6.9% in this study compares favorably with that in other studies, which indicated that operative mortality ranged from 13% to 31% (3-5). Alsoufi and colleagues (3) failed to find the independent risk factors for perioperative mortality after TVS. Gersh and colleagues (1) found that preoperative NYHA class IV was the only significant variable influencing hospital mortality. In our study, we found that old age, preoperative renal failure, postoperative renal failure, postoperative pulmonary complications, and postoperative stroke are risk factors for perioperative mortality with univariate analysis, but only postoperative renal failure and stroke are independent risk factors on multivariable analysis. As for NYHA class, the low incidence of NYHA class IV compared with that in other studies did not reach statistical significance (3, 6).
There are reports describing short and long-term survivals for surgery (3, 7, 8). Alsoufi and colleagues (3) presented that the actuarial survival rates were 75% and 61% at 5, 10 yr after TVS. Galloway and colleagues (7) showed that patients undergoing TVS had a 5-yr actuarial survival of 62%. Yilmaz and colleagues (8) reported that the actuarial survival rates were 85%, 72%, and 48% at 5, 10, and 15 yr after TVS. In our study we found a 5-yr and 10-yr survival of 87% and 84%, respectively. We believe that improved perioperative care, including increased experience with complex surgical procedures and improved myocardial protection, and improved postoperative care, including treatment of congestive heart failure and decreased valve-related complications, contributed to the very good long-term survival rates observed in our study. In addition, the low incidence of NYHA class IV in our patient population may be another important factor in favor of better long-term survival rates.
Unfortunately, the experience of prosthetic valve replacement in the tricuspid position is limited and the optimal choice for the tricuspid valve is still controversial. Some authors point out that tricuspid repair or replacement had no significant effect on mortality after surgery (9). On the other hand some authors report that replacement is associated with a high risk and a late mortality (10, 11). However, we did not find tricuspid valve replacement as an independent risk factor.
In addition, 20 cases of reoperation were performed. Ten-year freedom from reoperation was 84% and was thought to be lower than 92% in another study (3). However, we did not find a reoperation as an risk factor of late mortality.
Prosthetic valve-related complications have been reported to be more common in patients undergoing TVS compared with single valve replacement (1, 5). In our study 86% of prosthetic valves were mechanical. Although we did not compare valve morbidities among patients undergoing single valve surgery versus TVS in our institute, our valve-related complications in this study were comparable to the reported rate after single valve replacement in other recently published series (12, 13). Addtionally, prosthetic valve endocarditis was found to be the only risk factor of late mortality on the univariate and multivariate analysis, as we had anticipated.
Thromboembolism and bleeding complications are the major problems due to anticoagulation (2, 8, 14). Alsoufi and colleagues (3) reported a probability of freedom from thromboembolism of 88% at 10 yr and from anticoagulation-related hemorrhage of 83% at 10 yr. In our study these rates were 89% and 82%, respectively, for the same period. Current international normalized ratio target levels of 2.5 to 3.5 are advocated after mitral valve replacement using a mechanical prosthesis, similarly we recommend international normalized ratio target levels of 2.5 to 3.0 after multiple valve replacement.
TVS may be required in patients with rheumatic valve disease, prosthetic valve dysfunction, and endocarditis. TVS procedures are complex, and major cardiac reconstruction is often needed, increasing the complexity of these procedures. Nonetheless, short-term and long-term outcomes have improved when compared with older reports. We found acceptable rates of perioperative mortality for TVS with good long-term results. The majority of TVS patients are in NYHA class I or II during follow-up, and long-term valve-related complications rates are similar to patients undergoing single valve replacement. The good results after TVS in patients with advanced valvular heart disease justifies aggressive surgical therapy in these patients.
However, a number of limitations are inherent to this analysis design: a retrospective single center study with a relatively small sample size and the lack of information about the preoperative pulmonary artery pressure in our patients as this preoperative factor may influence the surgical outcome. Another limitation of this study is as follows: several surgeons were involved and we cannot expect the same surgical technique. Different patient characteristics and various kinds of substituted valves make a heterogenous group. These may influence outcome and prognostic factors.
In conclusion, postoperative renal failure and stroke are significantly related with operative mortality. To alleviate the operative mortality, we have to take account of reducing the incidence of postoperative complications. Triple valve surgery, regardless of reoperation and tricuspid valve replacement, which are considered as risk factor of mortality, results in acceptable long-term survival.
Figures and Tables
Fig. 2
Long-term freedom from morbid events. (A) Long-term freedom from prosthetic valve endocarditis. (B) Long-term freedom from thromboembolism. (C) Long-term freedom from anticoagulation-related hemorrhage. (D) Long-term freedom from reoperation

References
1. Gersh BJ, Schaff HV, Vatterott PJ, Danielson GK, Orszulak TA, Piehler JM, Puga FJ, Pluth JR, McGoon DC. Results of triple valve replacement in 91 patients: perioperative mortality and long-term follow-up. Circulation. 1985. 72:130–137.

2. Carrier M, Pellerin M, Bouchard D, Perrault LP, Cartier R, Hebert Y, Basmadjian A, Page P, Poirier NC. Long-term results with triple valve surgery. Ann Thorac Surg. 2002. 73:44–47.

3. Alsoufi B, Rao V, Borger MA, Maganti M, Armstrong S, Feindel CM, Scully HE, David TE. Short- and long-term results of triple valve surgery in the modern era. Ann Thorac Surg. 2006. 81:2172–2177.

4. Brown PS Jr, Roberts CS, McIntosh CL, Swain JA, Clark RE. Late results after triple-valve replacement with various substitute valves. Ann Thorac Surg. 1993. 55:502–508.
5. Mullany CJ, Gersh BJ, Orszulak TA, Schaff HV, Puga FJ, Ilstrup DM, Pluth JR, Danielson GK. Repair of tricuspid valve insuffiency in patients undergoing double (aortic and mitral) valve replacement. Perioperative mortality and long-term (1 to 20 years) follow-up in 109 patients. J Thorac Cardiovasc Surg. 1987. 94:740–748.
6. Akay TH, Gultekin B, Ozkan S, Aslim E, Saritas B, Sezgin A, Aslamaci S. Triple-valve procedures: impact of risk factors on midterm in a rheumatic population. Ann Thorac Surg. 2006. 82:1729–1734.

7. Galloway AC, Grossi EA, Baumann FG, LaMendola CL, Crooke GA, Harris LJ, Colvin SB, Spencer FC. Multiple valve operation for advanced valvular heart disease: results and risk factors in 513 patients. J Am Coll Cardiol. 1992. 19:725–732.

8. Yilmaz M, Ozkan M, Boke E. Triple valve surgery: a 25-year experience. Anadolu Kardiyol Derg. 2004. 4:205–208.
9. Wei J, Chang CY, Lee FY, Lai WY. De Vega's semicircular annuloplasty for tricuspid valve regurgitation. Ann Thorac Surg. 1993. 55:482–485.

10. Kaul TK, Mercer JL. Tricuspid valve replacement: factors influencing early and late mortality. Thorac Cardiovasc Surg. 1990. 38:229–235.

11. Scully HE, Armstrong CS. Tricuspid valve replacement. Fifteen years of experience with mechanical prosthesis and bioprosthesis. J Thorac Cardiovasc Surg. 1995. 109:1035–1041.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download