Abstract
Pulmonary adenocarcinoma is a common malignancy that often involves calcification; however, bone formation in primary lung adenocarcinoma is extremely rare. In ten cases of primary pulmonary adenocarcinoma with heterotopic ossification, we detected immunoreactivity against TGF-β1, osteopontin, osteocalcin and Runx2 in the fibroblastic stroma and tumor cells within the area of ossification. Our results suggest that in primary pulmonary adenocarcinoma, heterotopic ossification occurs via intramembranous bone formation. To our knowledge, only 11 other cases of pulmonary adenocarcinoma with heterotopic ossification have been reported. Here, we present ten cases of pulmonary adenocarcinoma showing heterotopic ossification with a description of previously published results and the histogenesis of heterotopic bone formation.
Pulmonary adenocarcinoma is a common malignancy that is prone to calcification, although bone formation in primary lung adenocarcinoma is extremely rare. Heterotopic ossification in primary lung adenocarcinoma was first described by McLendon et al. in 1985 (1). To our knowledge, however, only 11 cases of pulmonary adenocarcinoma with heterotopic ossification have been reported in the literature, and the isolated cases described in these reports involved ossification in bronchogenic carcinoma (1-11). Thus, the mechanism of heterotopic ossification in pulmonary adenocarcinoma is unknown.
Here, we describe the clinicopathologic features of ten cases of pulmonary adenocarcinoma with heterotopic ossification in addition to a review of previously published data and a discussion on the histogenesis of bone formation.
Ten cases of pulmonary adenocarcinoma with heterotopic ossification diagnosed in the Department of Pathology at the Samsung Medical Center in Seoul, Korea, between 2003 and 2007 were considered (Table 1). The surgical procedures performed on the patients included nine lobectomies and one bilobectomy. All tumors less than 5 cm were completely embedded in paraffin. Hematoxylin and eosin (H&E)-stained slides were then prepared for analysis by two experienced pulmonary pathologists to confirm the diagnosis and to assess multiple histologic features. Each patient's medical records and surgical pathology reports were also reviewed. Immunohistochemical staining was performed in patients 1-8 using 4-µm-thick tissue sections and a Bond Polymer Intense Detection System (VisionBioSystems, VIC, Australia) according to the manufacturer's instructions with minor modifications. In brief, each formalin-fixed and paraffin-embedded section was deparaffinized with Bond Dewax Solution (VisionBioSystems), and subjected to antigen retrieval using Bond ER Solution (VisionBioSystems) at 100℃ for 30 min. The endogenous peroxidase was subsequently quenched by incubation with hydrogen peroxide for 5 min. The sections were then incubated for 15 min at room temperate with thyroid transcription factor-1 (TTF-1) (8G7G3/1; 1:3,000; Dako, Glostrup, Denmark), p53 (DO-7; 1:1,000; Novocastra, Newcastle Upon Tyne, U.K.), Ki-67 (MIB-1; 1:500; Dako), osteopontin (OP3N; 1:400; Novocastra), osteocalcin (FL-100; 1:50; Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), Runx-2 (1D2; 1: 1,000; ABNOVA, Taipei, Taiwan) and transforming growth factor-β1 (TGF-β1) (rabbit polyclonal; 1:25; NeoMarkers, Fremont, CA, U.S.A.) using a biotin-free polymeric horseradish peroxidase-linker antibody conjugate system in a Bond-max automatic slide stainer (VisionBioSystems), and visualized using a 3,3'-diaminobenzidine (DAB) solution (1 mM DAB, 50 mM Tris-HCl, pH 7.6, and 0.006% H2O2). The slides were counterstained with hematoxylin. Formalin-fixed paraffin-embedded human gallbladder tissue for osteopontin, kidney for osteocalcin, and placenta for Runx2 and TGF-β1 were used as positive controls. The intensity of cytoplasmic staining for osteopontin, osteocalcin and TGF-β1 and nuclear staining for Runx2 was scored as follows; 0 (negative), + (weak), 2+ (moderate), and 3+ (strong). The signal was also regarded as positive for osteopontin, osteocalcin, Runx2, TGF-β1, TTF-1, and p53 if more than 10% of the specific cell population was immunostained. The Ki-67 labeled index values are given as percentages (%).
Chi-square and Fisher exact tests were used to evaluate the association between the expression of TGF-β1, osteopontin, osteocalcin and Runx2 for each the tumor and the fibroblastic stroma. Statistical significance in this study was considered at P<0.05. All reported p values were 2-sided. All analyses were performed with SPSS software (version 15.00, SPSS, Chicago, IL, U.S.A.).
The clinical features of ten cases of pulmonary adenocarcinoma with heterotopic ossification are listed in Table 1. The age of the patients ranged from 57 to 70 yr (mean 62.4 yr), and the male:female ratio was 4:6. Eight patients were non-smokers, while the remaining two (patients 2 and 5) had stopped smoking 10 and 15 yr earlier, respectively. The initial chief complaints of the patients were largely asymptomatic. Radiologically, radiopaque shadows in the center of the lung mass indicating calcification or ossification were found in nine of ten patients (all except patient 1). Due to the high mineral densities observed in some of the cases, hamartoma or tuberculosis was suspected. A trabecular pattern was observed in the center of the lung mass in patient 2, suggesting intratumoral ossification (Fig. 1). Nine of the patients received lobectomies while patient 2 received a bilobectomy, including right upper and middle lobes with dissection of the mediastinal lymph nodes. Neoadjuvant chemotherapy and concurrent chemoradiotherapy were administered in patient 6 and 7, respectively, while adjuvant chemotherapy was administered in patient 9.
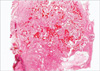
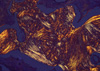
Our pathologic findings for each of the ten patients are listed in Table 2. The tumor, which initially appeared to be solid gray-white solid masses, had a granular appearance during sectioning, indicating calcification or ossification. The tumors, which ranged in diameter from 2 to 7 cm (mean 3.8 cm), were located in the right upper lobe in four patients, the left lower lobe in three patients, the right lower lobe in two patients, and in the right middle lobe in one patient. Histologically, the tumor pattern was papillary-tubular in eight patients, papillary in one patient (patient 3), and solid-tubular pattern in one patient (patient 6). The tumors were moderately differentiated in all cases except case 6, which exhibited poor differentiation. Mitotic figures were frequently observed in the tumor cells, and all of the tumors contained fragments of osseous tissue within the abundant central fibroblastic stroma (Fig. 2). Dense connective tissue was present in the area of ossification, but was absent from the periphery of the cancer (where ossification was not observed). In patient 8, occasional psammoma bodies were observed in the tumor papillae. Cellular atypia was absent from the stromal fibroblastic cells, osteoblasts, and osteocytes (Fig. 3). Minimal tumor necrosis (<5% of the tumor area) was present in patients 2, 6, 7, and 8; however, no necrosis was found in the ossification area. In patient 1, chronic inflammation was detected in the fibroblastic stroma; however, none of the other patients exhibited a mucin pool, inflammation, or preexisting calcification of the fibrous stroma in the area of ossification. Hyaline cartilage formation and a bluish chondroid stroma were absent in all patients. The size of the ossification area ranged from 0.05 to 2 cm (mean 0.78 cm). A tiny bony trabecula measuring 0.05 cm in length was observed in patient 1. Within the osseous tissue, osteoid formation with calcification and fully mature trabecular bone having osteocytes, osteoblastic rimming, and lamellation were observed. Lamellation of the trabecular bone under polarizing light was observed in eight of ten patients (Fig. 4), while rows of osteoblasts were seen along the edge of the osseous trabeculae, in all ten patients. Evident marrow spaces were observed in six of ten patients, while osteoclastic activity suggesting bone remodeling was found in five of ten patients. Metastasis of the peribronchial lymph nodes (N1 nodal stage) in patients 2 and 6, and the mediastinal (N2) lymph nodes in patient 7 was present, but bone formation was not detected.
Our immunohistochemistry are listed in Table 3. The tumor and fibroblastic stromal cells reacted with antibodies against TGF-β1, osteopontin, osteocalcin and Runx2 (Fig. 5A, D). The intensity of TGF-β1 and Runx2 staining was the same in the tumor and fibroblastic stromal cells. The immunoreactivity against osteopontin and osteocalcin was the stronger in the tumor and the fibroblastic stromal cells, respectively. The intensity of osteopontin was more significantly strong in the fibroblastic stroma of ossification area compared to the non-ossification (P=0.029). However, there was no significant difference in those of osteocalcin (P=0.097). The tumor cells also reacted with TTF-1 in all cases. Positive collagen type IV immunostaining was observed in patient 3 in the fibroblastic stromal cells. Positive reactivity for p53 was observed in six of eight patients (75%), and the index of positivity in the tumor cells ranged from 30 to 90% (mean 55.8%). The Ki-67 labeled proliferating index value for the tumor cells ranged from 1 to 35% (mean 11.3%); however, in the fibroblastic stromal cells, the index value was 0% in all cases.
All ten patients were alive during the follow-up period (4-52 months, mean 22.8 months). Two patients (patients 2 and 3) had recurrent remnants in the right lower lobe and at the lobectomy stump area 16 and 30 months after surgery, respectively, and chemotherapy was administered.
Intratumoral calcification has been observed in various neoplasms. In fact, benign heterotopic bone formation within neoplasms has been sporadically reported in the kidney, liver, gastrointestinal tract, breast, thyroid, skin, and soft tissues. In contrast to dystrophic calcification, which frequently occurs in necrotic areas of cancer, heterotopic ossification within primary lung carcinomas is extremely rare. Although bronchial carcinoid is one such calcifying or ossifying tumor, a review of the literature revealed only 11 other cases of heterotopic ossification in pulmonary adenocarcinoma and two cases in pulmonary squamous cell carcinoma (1-13). All previously published cases of pulmonary adenocarcinoma with heterotopic ossification are listed in Table 4. In those cases, the patients ranged in age from 49 to 76 (mean 62.5 yr) and the male:female ratio was 7:4. One of the 11 patients was American, while the others were Japanese. The predominant histologic type of the tumors was moderately differentiated adenocarcinoma with a dense fibrous stroma located at the peripheral lung zone with pleural dimpling. Two patients also showed osteoplastic metastasis (2, 5).
Although intrapulmonary calcification is frequently observed radiologically, distinguishing calcification from ossification is difficult. In some cases in our series, the lesion was interpreted as benign with hamartoma or tuberculosis. However, ossification can be differentiated radiologically from calcification when a trabecular or reticular pattern is detected by CT (14, 15).
Pathologic calcification implies abnormal deposition of calcium salts. This is a common process that occurs under a variety of pathologic conditions, such as hypercalcemia, necrosis, extracellular mucin and chronic inflammation. In contrast, ossification is a more complex process involving osteoblasts and osteocytes. Several theories exist regarding the mechanism of intratumoral calcification and ossification, including calcification in the presence of degenerative or necrotic tissues (dystrophic calcification), entrapment of preexisting calcified scar tissue or granulomatous disease, and calcium deposition within the tumor as a result of mucus production by tumor cells (16, 17).
Two means of osteogenesis are known: intramembranous bone formation and endochondral bone formation. Both processes feature the transformation of a primary trabecular network into mature bone, but differ in terms of the starting point. Intramembranous bone formation involves the transformation of a mesenchymal template into bone, while endochondral ossification involves the replacement of a preexisting hyaline cartilage template into bone. The sequence of events in intramembranous bone formation includes the formation of mesenchymal condensations via a process controlled by Wnt, hedgehog, fibroblast growth factor, and TGF-β family polypeptides; differentiation of the mesenchymal cells into osteoblasts, deposition of the bone matrix by the osteoblasts, mineralization and differentiation of osteocytes by interstitial growth under the control of the transcription factors Cbfa1/Runx2 and osterix. Cbfa1/Runx2 is the earliest and more specific indicator of osteogenesis and its expression is induced by bone morphogenic protein 7, followed by the expression of osteocalcin and osteopontin. Osteopontin and osteocalcin are non-collagenous multifunctional glycoproteins routinely found in mineralized tissue, where it is believed to play an integral role in the cellular response to mechanical stimuli. These proteins also play a role in the initial formation of the matrix, depending on the mode of ossification and bone morphogenesis (18, 19). A progressive increase in the amount of osteopontin in the membranous bone matrix during the maturation of new bone was previously reported (20). Osteocalcin is a specific secretory protein expressed only in terminally differentiated osteoblasts under the control of Cbfa1/Runx2.
Our Immunohistochemical results provide pertinent information concerning the histogenesis of heterotopic ossification. We propose that the heterotopic ossification of primary pulmonary adenocarcinomas occurs via intramembranous bone formation, and is induced by the tumor cells. Our reasoning is as follows; no necrosis, mucin pool, or inflammation of the fibroblastic stroma in the area of ossification was observed in any of the patients, except patient 1, who exhibited chronic inflammation of the fibroblastic stroma; preexisting calcification with hyaline cartilage formation or a bluish chondroid stroma was not observed in the areas of ossification; the level of immunoreactivity against TGF-β1, osteopontin, osteocalcin and Runx2 was highest in the tumor cells and the fibroblastic stroma; and osteopontin was more expressed in fibroblatic stroma surrounding the area of ossification compared to the area of non-ossification, which indicate osteopontin has more osteoblastic activity in fibroblastic stroma surrounding the area of bony trabecullae. Still, we cannot completely exclude the effect of the neoadjuvant chemotherapy applied to patient 6 and neoadjuvant concurrent chemoradiotherapy to patient 7, and patient 1 exhibited chronic inflammation of the fibroblastic stroma and a tiny bony trabecula. However, none of the other patients showed necrosis with cholesterol granuloma or inflammation, the aggregation of foamy macrophages, in the area of ossification.
The stromal response should be differentiated from true sarcomatous participation in the tumors. Pulmonary carcinosarcomas show a distinct malignant stroma and osteoid formation. As far as the present tumors are concerned, the possibility of a carcinosarcoma is ruled out by the lack of malignancy in the fibroblastic stroma and osteoid. The observed intratumoral ossification consisted of fully matured bone elements with no cellular atypia, and mitosis was not detected in the stromal fibroblastic cells. Additionally, the Ki-67 labeled proliferating index value was 0% in the stromal fibroblastic cells and bony trabeculae. Therefore, we presume that the osseous elements were produced through benign reactive or metaplastic processes.
In conclusion, we characterized ten cases of primary pulmonary adenocarcinoma with heterotopic ossification. Although, distinguishing calcification from ossification radiologically is difficult, ossification can be recognized based on the presence of a trabecular or reticular pattern. We detected immunoreactivity against TGF-β1, osteopontin, osteocalcin and Runx2 in the tumor cells and fibroblastic stroma surrounding the area of ossification. Our results indicate that heterotopic ossification in primary pulmonary adenocarcinoma occurs via an intramembranous bone formation and osteopontin has more osteoblastic activity in fibroblastic stroma surrounding the area of bony trabecullae. However, additional studies with a greater number of cases are needed to elucidate the mechanisms of intratumoral osteogenesis and osteogenic metastasis and their prognosis.
Figures and Tables
Fig. 1
Chest CT showing a radiodense trabecular pattern (arrow) in the center of the lung mass (patient 2).
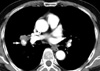
Fig. 3
(A) Microscopic images showing moderately differentiated adenocarcinomas with mature bony trabeculae in the abundant fibroblastic stroma. (B) Microscopic images showing spindled fibroblastic cells and bony trabeculae with osteoblastic rimming and osteocytes. Cellular atypia is absent (H&E staining, [A] ×40; [B] ×200).

Fig. 5
Immunohistochemical staining showing the presence of osteopontin (A), TGF-β1 (B), osteocalcin (C), and Runx2 (D) in the tumor and fibroblastic stromal cells. Polymer method, ×200.
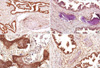
Table 3
Immunohistochemical results of pulmonary adenocarcinoma with heterotopic ossification (patients 1-8)

References
1. McLendon RE, Roggli VL, Foster WL Jr, Becsey D. Carcinoma of the lung with osseous stromal metaplasia. Arch Pathol Lab Med. 1985. 109:1051–1053.
2. Yoshida K, Morinaga S, Gemma A, Shimosato Y, Tsuchiya R, Eguchi K. Adenocarcinoma with stromal bone metaplasia of the lung. Jpn J Lung Cancer. 1988. 28:87–92.

3. Miyata S, Nakagawa T, Maeda S, Miwa A, Kitagawa M, Takashima T. A case of adenocarcinoma of the lung with calcification on chest CT. Jpn J Lung Cancer. 1988. 28:107–111.

4. Fukuse T, Koh T, Okumura N, Kuwabara M, Suzuki Y. A case of primary lung cancer with stromal ossification. Jpn J Lung Cancer. 1990. 30:267–272.

5. Hayakawa K, Murata O, Ishizeki K, Saito Y, Hasegawa M, Yamakawa M, Mitsuhashi N, Shimizu K, Niibe H. A case of pulmonary ossified adenocarcinoma with marked osteoplastic bone metastasis. Lung Cancer. 1997. 18:265–268.

6. Tsuchiya T, Nishimura Y, Funada Y, Nakajima T, Hozumi A, Kotani Y, Nishiuma T, Matsumoto K, Ohbayashi C, Yokoyama M. Pulmonary adenocarcinoma with central ossification. Nihon Kokyuki Gakkai Zasshi. 2000. 38:283–287.
7. Hara H, Iwabuchi K, Shinada J, Yoshimura H, Kameya T. Pulmonary adenocarcinoma with heterotopic bone formation. Pathol Int. 2000. 50:910–913.

8. Hosoda H, Izumi H, Atarashi K, Shinohara N, Sunamori M. Lung adenocarcinoma with stromal ossification. Jpn J Lung Cancer. 2002. 42:51–54.

9. Usami N, Yoshioka H, Mori S, Imaizumi M, Nagasaka T, Ueda Y. Primary lung adenocarcinoma with heterotopic bone formation. Jpn J Thorac Cardiovasc Surg. 2005. 53:102–105.

10. Ueshima Y, Kurioka H, Yamada R, Takumi C, Hiraoka N, Ono S. Stromal bone formation by lung adenocarcinoma. Nihon Kokyuki Gakkai Zasshi. 2005. 43:523–526.
11. Kato F, Iwasaki A, Miyoshi T, Nakajima H, Hirayama T, Yamamoto S, Hiratsuka M, Shiraishi T, Shirakusa T, Hayashi H. A case of primary adenocarcinoma of the right lung with ossification. J Jpn Assoc Chest Surg. 2006. 20:856–859.

12. Good CA, McDonald JR. Roentgenologic evidence of calcification in a peripheral bronchogenic carcinoma. Proc Staff Meet Mayo Clin. 1956. 31:317–321.
13. Flanagan P, McCracken AW, Cross RM. Squamous carcinoma of the lung with osteocartilaginous stroma. J Clin Pathol. 1965. 18:403–407.

14. Cooney T, Sweeney EC, Luke D. Pulmonary carcinoid tumours: a comparative regional study. J Clin Pathol. 1979. 32:1100–1109.

15. Beauchamp NJ, Pizer E, Hruban RH, Fishman EK. Ossification of a rectal tumor: CT evaluation. J Comput Assist Tomogr. 1997. 21:671–673.
16. Mahoney MC, Shipley RT, Corcoran HL, Dickson BA. CT demonstration of calcification in carcinoma of the lung. AJR Am J Roentgenol. 1990. 154:255–258.

17. Kim WH, Kim YI. Ossification of the N-methyl-N'-nitro-N-nitrosoguanidine-induced small intestine adenocarcinomas in rats. J Korean Med Sci. 1991. 6:308–312.

18. Perrien DS, Brown EC, Aronson J, Skinner RA, Montague DC, Badger TM, Lumpkin CK Jr. Immunohistochemical study of osteopontin expression during distraction osteogenesis in the rat. J Histochem Cytochem. 2002. 50:567–574.

19. Koeneman KS, Kao C, Ko SC, Yang L, Wada Y, Kallmes DF, Gillenwater JY, Zhau HE, Chung LW, Gardner TA. Osteocalcin-directed gene therapy for prostate-cancer bone metastasis. World J Urol. 2000. 18:102–110.

20. Pinero GJ, Farach-Carson MC, Devoll RE, Aubin JE, Brunn JC, ButlerWT . Bone matrix proteins in osteogenesis and remodelling in the neonatal rat mandible as studied by immunolocalization of osteopontin, bone sialoprotein, alpha 2HS-glycoprotein and alkaline phosphatase. Arch Oral Biol. 1995. 40:145–155.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download