Abstract
It has been suggested that the patients with Cushing's disease secondary to pituitary macroadenomas (>10 mm) have higher basal adrenocorticotropic hormone (ACTH) levels, which are less suppressible on high-dose dexamethasone suppression tests (HDDST). We compared the clinical and biochemical characteristics of patients with macroadenomas (N=7) and microadenomas (N=23) who were diagnosed at Samsung Medical Center in Korea between 1996 and 2006. Basal morning plasma ACTH levels were 101.5±23.2 pg/mL for macroadenoma patients and 83.6±11.1 pg/mL for microadenoma patients (mean±SEMs) (p=0.44). Morning serum cortisol levels were 26.8±3.2 µg/dL for macroadenoma patients and 29.5±2.9 µg/dL for microadenoma patients (p=0.77). The proportion of patients who showed suppressibility on HDDST was almost identical in the two groups (71.4% [5/7] for macroadenoma patients vs. 72.7% [16/22] for microadenoma patients, p=1.00). Furthermore, the remission rate with trans-sphenoidal surgery was similar between the two groups (100% [5/5] for macroadenoma patients vs. 73.3% [11/15] for microadenoma patients, p=0.53). Thus, tumor size is not a major determinant of hormone secretion or clinical outcomes in patients with Cushing's disease.
Cushing's disease is characterized by adrenal hypersecretion of glucocorticoids secondary to overproduction of adrenocorticotrophic hormone (ACTH) by a corticotroph adenoma in the pituitary gland. Apart from iatrogenic Cushing's syndrome, Cushing's disease is the most common cause of hypercortisolism, comprising approximately 70% of all such cases (1). The incidence of Cushing's disease is estimated to be 0.7 to 2.4 cases per million people per year. The disease occurs more commonly in women than in men (1, 2). In the majority of cases, ACTH-secreting pituitary adenomas are small (less than 10 mm in diameter) and confined within the sella turcica. However, 4-10% of patients present with larger tumors (more than 10 mm in diameter) (3). These larger tumors sometimes cause symptoms due to mass effect before there are full-blown endocrine manifestations.
For patients with corticotroph adenomas, the set point of ACTH suppressibility to glucocorticoids is altered, and larger amounts of exogenous glucocorticoids are necessary to suppress plasma ACTH levels. Therefore, pituitary corticotroph adenomas are characterized by blunting of cortisol feedback to low-dose dexamethasone testing, but the plasma ACTH levels are suppressible with administration of high dose dexamethasone (4). However, it has been reported that corticotroph macroadenomas are often associated with higher ACTH levels and less glucocorticoid suppressibility on high-dose dexamethasone administration, whether assessment is performed through measurement of urinary free cortisol excretion or through serum cortisol levels (5, 6). Moreover, it has also been reported that macroadenomas are more refractory to surgical treatment and show a more unfavorable prognosis than microadenomas (7, 8).
The aim of this study was to compare the clinical and biochemical characteristics of Cushing's disease patients with corticotroph macroadenomas with those of patients with microadenomas.
This study included 30 patients (5 male, 25 female; mean age 38.7 yr [range: 13-73 yr]) with pituitary-dependent ACTH-secreting adenomas. All patients were diagnosed at Samsung Medical Center between 1996 and 2006. Of the 30 patients, 23 (76.7%) were diagnosed with pituitary corticotroph microadenomas, and the remaining 7 patients (23.3%) were diagnosed with macroadenomas by sella magnetic resonance imaging (MRI). None of the macroadenoma patients was male, and 5 of the 23 microadenoma patients were male.
The diagnosis of Cushing's disease was based on characteristic signs and symptoms, laboratory findings, sella MRI, inferior petrosal sinus samplings (IPSS), and histopathology. Hormonal assays were performed according to standardized clinical procedures. At entry into the study, all patients were admitted to the hospital, whereupon their basal plasma ACTH and serum cortisol concentrations were measured at 0800h to 0900h. Most patients (26/30) had their urine collected for 24 hr to determine the 24-hr urinary free cortisol, along with the urine creatinine. Dynamic tests were done for all patients. That is, all but one patient underwent the high-dose dexamethasone suppression tests (HDDST), and the remaining patient underwent the corticotropin-releasing hormone (CRH) stimulation test. The HDDST was performed using one of the following methods: 1) oral administration of 2 mg of dexamethasone every 6 hr for 2 days (N=25); 2) suppression of the serum cortisol during 7 hr of intravenous dexamethasone administration (N=5); or 3) 8mg overnight dexamethasone suppression test (N=1). The serum and urine cortisol levels after HDDST were defined as "responsive" if the serum values fell by more than 50% of the baseline and if the urine values fell by more than 90% of the baseline. Biochemical assays were also used to measure the preoperative basal levels of hGH, IGF-1, TSH, free or total T4, FSH, LH, and prolactin. Twenty patients underwent preoperative combined pituitary stimulation testing, principally those in the macroadenoma group. IPSS was performed before and after administration of CRH stimulation in 17 patients with Cushing's disease because either their MRI findings showed no definite mass with gadolinium enhancement or their biochemical tests were equivocal. Sampling of the plasma ACTH levels was performed at baseline and at 1, 3, 5, 10, 15, and 30 min after intravenous ovine CRH (0.1 mg) injection. An increased ratio (>2) of the inferior petrosal:peripheral vein sample at baseline and a peak inferior petrosal:peripheral vein ACTH ratio of ≥3 after CRH injection confirmed the presence of an ACTH-secreting pituitary tumors. IPSS demonstration of a right-to-left venous ACTH gradient of less than 0.7 or greater than 1.4 was considered to represent a lateralization to the left or right, respectively.
Surgical modalities for treating Cushing's disease included endoscopic trans-sphenoidal surgery (TSA) (N=22), stereotactic gamma knife surgery (N=6), and open craniotomy (N=1); one patient was lost to follow-up without receiving any treatment. If patients were not cured by their initial treatment, then they underwent a second treatment using external radiation therapy (N=1) or gamma knife surgery (N=4). All but one patient underwent TSA by a single surgeon through trans-septal or endonasal access. If the tumor was identified by preoperative MRI or in the operation field, then gross total tumor removal was performed. If the tumor was not localized on the preoperative MRI, but IPSS showed an ACTH level gradient, the first exploration was made on the side with the higher ACTH gradient. If no tumor was identified upon exploring the entire gland, then partial hemihypophysectomy was performed either on the side that showed ACTH dominance on IPSS testing or on the side where tumor was likely to exist as determined by intraoperative frozen biopsy. Gamma knife surgery was indicated in case where the lesion was not amenable to TSA, such as in case of intrasellar or cavernous invasion.
Basal ACTH and cortisol levels were measured on postoperative day 2 in all patients who underwent TSA; some patients underwent repeat basal hormone sampling within 1 week after operation. Patients were assessed for clinical remission at 6 months after TSA, with remission defined as being free of symptoms caused by excessive glucocorticoid hormone and either 1) dependency on glucocorticoid substitutions or 2) biochemical eucortisolemia (morning serum cortisol from 6 to 26 µg/dL) without hormone replacement. Among the patients who underwent TSA (N=22), 20 patients were followed for at least 6 months (range: 10 to 101 months, mean 41 months). Persistent Cushing's disease was defined as failure to fulfill the biochemical criteria for remission at 6 months after the first operation and along with necessity of a second intervention. Relapse was defined as recurrence of clinical signs and symptoms in the presence of basal serum cortisol levels increased above the upper limit of normal and non-suppressibility of cortisol on low dose dexamethasone suppression testing (0.5 mg of dexamethasone every 6 hr for 2 days).
Statistical analysis was performed using SPSS 13.0 for Windows (SPSS, Inc., Chicago, IL, U.S.A.). Data are depicted as means±SEMs for continuous variables and as percents for dichotomous variables. The mean differences between macroadenomas and microadenomas were compared using the Mann-Whitney test, and differences between the observed frequencies were compared using Fisher's exact test. Comparisons with p values less than 0.05 were considered statistically significant.
At baseline, the mean ages of patients with macroadenomas and microadenomas were 35.7±3.7 and 39.6±3.3 yr, respectively (means±SEMs) (p=0.74). Five of 30 patients were male, and all of them had microadenomas. There were no clinical signs or symptoms that differentiated macroadenomas from microadenomas before sella MRI was performed, including visual field defects (data not shown). Fifty-three percent of all patients (52% [12/23] microadenoma vs. 57% [4/7] macroadenoma) were diagnosed with obesity, which was defined as a body mass index (BMI) more than 25 kg/m2. Fifty percent of all patients (43% [10/23] microadenoma vs. 71% [5/7] macroadenoma) had diabetes based on previous medication history or on fasting plasma glucose levels equal to or greater than 126 mg/dL on admission. Characteristically, the majority of patients (83%, 25/30) had high blood pressure, with a statistically significant difference between the groups (96% [22/23] microadenoma vs. 43% [3/7] macroadenoma, p=0.006) (data not shown).
Early morning basal plasma ACTH and serum cortisol levels were available for all patients; the urine free cortisol level was measured in 26 patients. Although patients with macroadenomas showed a tendency to have higher ACTH levels, there was no statistically significant difference between the two groups (101.5±23.2 pg/mL for macroadenomas vs. 83.6±11.1 pg/mL for microadenomas, p=0.44). Serum cortisol and urine free cortisol levels also showed no statistically significant difference. Moreover, there was no linear correlation between maximal tumor diameter and preoperative basal ACTH, cortisol, and urine free cortisol levels (data not shown). With regard to the cortisol to ACTH ratio, which is the degree of cortisol secretion for a given ACTH level, there was no statistically significant difference between the two groups (0.31±0.05 for macroadenomas vs. 0.47±0.09 for microadenomas, p=0.21) (Table 1).
After high dose dexamethasone administration, 72.4% (21/29) of patients showed suppressibility. However, there was no difference between the two groups with regard to the percentage of patients that manifested suppression (71.4% [5/7] for macroadenoma patients vs. 72.7% [16/22] for microadenoma patients, p=1.00) or with regard to the magnitude of suppression (65.6±11.2% for macroadenoma patient vs. 65.9±5.3% for microadenoma patients, p=0.82) (Table 2). Of the 21 patients with microadenomas who were followed for at least 6 months, 15 patients underwent TSA, and the remaining 6 patients underwent gamma knife surgery. There was no statistically significant difference in long-term remission rates between the two groups (73.3% for TSA [11/15] vs. 100% [6/6] for gamma knife surgery, p=0.28) (data not shown).
Among the patients who underwent TSA (N=22), one microadenoma patient and one macroadenoma patient were excluded from the analysis because their follow-up periods were not sufficient to determine their remission status. Clinical and biochemical remission was observed in 16 of 20 patients (80%), including 11 of 15 microadenoma patients (73.3%) and all macroadenoma patients (5/5) (Table 2). Among the four patients with non-remission, three patients never achieved remission status. That is, they had persistent Cushing's disease; one patient relapsed 28 months after the first operation.
To elucidate differences between patients in the remission group and patients in the non-remission group, we compared the baseline and immediate postoperative hormone levels in the two groups. There were no differences between the preoperative basal ACTH and cortisol levels of the groups (ACTH: 90.2±17.0 pg/mL in the remission group vs. 84.2±5.7 pg/mL in the non-remission group, p=0.49; cortisol: 29.4±3.7 µg/dL in the remission group vs. 33.3±6.8 µg/dL in the nonremission group, p=0.68). We found no differences in the preoperative HDDST suppressibility between the remission group and the non-remission group (66.7% [10/15] for the remission group vs. 75.0% [3/4] for the non-remission group, p=1.00) (Table 3). Although the postoperative plasma ACTH levels did not show a statistical difference between the two groups, the serum cortisol level, which was measured within one week after TSA, revealed a marginal difference between the remission group and the non-remission group (8.6±3.6 µg/dL for the remission group vs. 14.3±5.9 µg/dL for the non-remission group, p=0.06) (Table 3).
We then determined if tumor mass still remained after operation, with complete tumor removal defined as no residual tumor mass on postoperative sella MRI and positive ACTH immunoreactivity in the tumor cells. Although no statistically significant difference was observed between the two groups, patients in the remission group tended to have complete tumor removal on imaging and biopsy, as compared with the nonremission group (64.3% [9/14] for the remission group vs. 25.0% [1/4] for the non-remission group, p=0.28) (Table 3).
In this study, 7 (22.6%) out of the 30 patients with Cushing's disease had macroadenomas. Although we took into account that our institution is a large tertiary center, the proportion of the patients with macroadenoma was somewhat higher than that of the previous reports. According to the report by the survey committee for endocrine disease in Korean Endocrine Society, out of the 87 patients with Cushing's disease, nine patients (10.3%) had macroadenomas (9). However, it was also reported that the true prevalence of Cushing's disease that's caused by corticotroph macroadenomas has been underestimated and the actual number maybe reach approximately 20% of all patients with Cushing's disease (10).
Previous studies have suggested that Cushing's disease caused by macroadenomas showed higher levels of basal ACTH and cortisol, and less suppressibility with high dose dexamethasone suppression testing as compared with microadenomas (5, 6). Woo et al. reported that the plasma ACTH and serum cortisol levels were significantly higher in the macroadenoma group than those in the microadenoma group (3). However, the cortisol-to-ACTH ratio showed relatively lower levels of cortisol for a given ACTH level in the macroadenoma group. Thus, they speculated that this was caused by the lowered biological activity of ACTH in the patients with macroadenoma because the ACTH assay that was used cross-reacts with the ACTH precursor POMC. Consequently, the proportion of the biologically active form of ACTH was lowered with the immunoassay that they used. Actually, Gibson et al. proposed that macroadenomas were poorly differentiated and had impaired processing of POMC to ACTH; moreover, they preferentially secrete the biologically inactive precursors of ACTH that could interfere with ACTH measurements and the secretion of which is not regulated like that of ACTH (11).
However, our results did not show any significant differences of the basal plasma ACTH, serum cortisol or the urine free cortisol levels between microadenomas and macroadenomas. Moreover, there were no linear correlations between the maximal tumor diameter and the ACTH, cortisol and urine free cortisol levels. We offer the following explanations for our results. First, our ACTH assay does not cross-react with POMC; therefore it was unlikely that the plasma ACTH levels in our results were falsely increased by biologically inactive ACTH precursors. Therefore, the cortisol to ACTH ratio was not lower in the macroadenoma group in our study. Second, although it did not reach statistical significance, the basal ACTH level in the macroadenoma group showed a tendency to be higher as compared with the microadenoma group. However, there was a wide range of the ACTH levels and there was considerable overlap between the groups, so it was difficult to predict whether the patient would be in the macroadenoma group or the microadenoma group with examining the preoperative ACTH levels. Third, it seems that the biological behavior and clinical outcomes of Cushing's disease were not determined by tumor size, and the cutoff point of 10 mm that divided the Cushing's disease into macroadenomas and microadenomas was thought to be somewhat arbitrary as is the case for thyroid carcinoma.
It was reported that macroadenomas showed more aggressive clinical behaviors and they were more refractory to treatments than microadenomas (7, 8). However, in our study, the long-term remission rate with TSA was not low for the macroadenoma patients and moreover, all five patients with macroadenoma were successfully treated by their first operation (73.3% [11/15] vs. 100% [5/5] for microadenomas and macroadenomas, respectively, p=0.53). The results of Pereira et al. are in accordance with our findings and they reported that although macroadenomas showed a tendency for delayed normalization of the serum cortisol level compared with microadenomas, the long-term cure rate of macroadenomas was not different from that of microadenomas (12). Also, the remission rate of Cushing's disease was higher in macroadenomas compared with microadenomas by the report from Korean Endocrine Society (32.7% [18/55] vs. 57.1% [4/7] for microadenomas and macroadenomas, respectively) (9). Instead, it seems that the surgical outcome was critically determined by complete surgical removal of the tumor mass rather than by the size of the mass. For the patients with microadenoma, 4 patients out of 16 were not cured after their first operation and they underwent an additional treatment modality. Among these four patients, all but one of these patients had a tumor mass that was poorly localized on the preoperative sella MRI and/or operation field, and it was difficult to accurately remove the tumor mass. Although it did not reach statistical significance, the patients with remission showed a tendency of complete tumor removal, which was defined as no residual tumor mass on postoperative sella MRI and positive ACTH immunoreactivity in the tumor cells, as compared with the non-remission group (Table 3).
The definition of a biochemical cure of Cushing's disease has been the subject of much controversy (7, 12-16). A variety of criteria have been used to assess the postoperative disease status. Some investigators have advocated performing provocative tests such as an over-night low-dose dexamethasone suppression test (17) or a CRH stimulation test (18), and other investigators have used non-provocative tests such as measuring the basal ACTH and cortisol levels or the 24-hr urine free cortisol concentration (12, 15); moreover, these tests were assessed at different time points, respectively. Rees et al. have proposed a more stringent requirement of a postoperative serum cortisol level of <1.8 µg/dL within 1 week after TSA as a definition of remission; with using this criterion, they reported a remission rate of 77% during a median of 6 yr follow-up (14). However, it appears that this value of less than 1.8 µg/dL of serum cortisol is too strict and some patients, who are in long-term remission, were above this threshold at their immediate postoperative periods. Indeed, in the present study, out of the 16 patients with remission, only five patients (31.3%) met this strict definition of less than 1.8 µg/dL of serum cortisol. Also, although the number of patients was limited, the post-operative serum cortisol level, which was measured within 1 week after TSA, was different between the remission group and the non-remission group (8.6±3.6 µg/dL vs. 14.3±5.9 µg/dL for the remission and non-remission groups, respectively, p=0.06). Therefore, somewhat higher immediate postoperative serum cortisol levels can be allowed to predict long-term remission, as compared with the values proposed by the previous reports. Actually, about one third of the patients with remission (5/14) showed a serum cortisol level of more than 5.0 µg/dL within 1 week after operation.
The main limitation of the present study was that the number of patients with macroadenoma was relatively small. Therefore, further studies are needed to confirm the biologic behavior and long-term prognosis of Cushing's disease according to the size of the tumor mass.
In conclusion, the pituitary Cushing's disease that was caused by macroadenomas was not different on the biochemical tests from the disease that was caused by microadenomas, including the basal hormone levels and the suppressibility with HDDST. Moreover, the clinical outcomes of TSA also showed no difference between the two groups. Instead, it appears that the surgical outcome was dependent on accurate tumor localization and complete mass removal.
Figures and Tables
Table 1
Comparisons of the baseline characteristics and basal hormone levels between patients with macroadenomas and patients with microadenomas

Table 3
Comparison of the baseline characteristics and postoperative hormone levels according to the treatment outcome with TSA
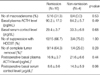
The data is expressed as means±SEM for continuous variables and as numbers (%) for the dichotomous variables.
*Complete tumor removal was defined as no residual tumor mass on the postoperative sella MRI and positive ACTH immunoreactivity in the tumor cells; †The postoperative basal hormone levels were measured within 1 week after TSA.
HDDST, high-dose dexamethasone suppression test; TSA, trans-sphenoidal approach; ACTH, adrenocorticotropic hormone.
References
1. Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing's syndrome. Lancet. 2006. 367:1605–1617.

2. Lindholm J, Juul S, Jørgensen JO, Astrup J, Bjerre P, Feldt-Rasmussen U, Hagen C, Jørgensen J, Kosteljanetz M, Kristensen L, Laurberg P, Schmidt K, Weeke J. Incidence and late prognosis of cushing's syndrome: a population-based study. J Clin Endocrinol Metab. 2001. 86:117–123.
3. Woo YS, Isidori AM, Wat WZ, Kaltsas GA, Afshar F, Sabin I, Jenkins PJ, Monson JP, Besser GM, Grossman AB. Clinical and biochemical characteristics of adrenocorticotropin-secreting macroadenomas. J Clin Endocrinol Metab. 2005. 90:4963–4969.

4. Newell-Price J, Trainer P, Besser M, Grossman A. The diagnosis and differential diagnosis of Cushing's syndrome and pseudo-Cushing's states. Endocr Rev. 1998. 19:647–672.

5. Katznelson L, Bogan JS, Trob JR, Schoenfeld DA, Hedley-Whyte ET, Hsu DW, Zervas NT, Swearingen B, Sleeper M, Klibanski A. Biochemical assessment of Cushing's disease in patients with corticotroph macroadenomas. J Clin Endocrinol Metab. 1998. 83:1619–1623.
6. Selvais P, Donckier J, Buysschaert M, Maiter D. Cushing's disease: a comparison of pituitary corticotroph microadenomas and macroadenomas. Eur J Endocrinol. 1998. 138:153–159.

7. Blevins LS Jr, Christy JH, Khajavi M, Tindall GT. Outcomes of therapy for Cushing's disease due to adrenocorticotropin-secreting pituitary macroadenomas. J Clin Endocrinol Metab. 1998. 83:63–67.

8. Cannavo S, Almoto B, Dall'Asta C, Corsello S, Lovicu RM, De Menis E, Trimarchi F, Ambrosi B. Long-term results of treatment in patients with ACTH-secreting pituitary macroadenomas. Eur J Endocrinol. 2003. 149:195–200.

9. The Korean Society of Endocrinology. The Survey Committee for Endocrine Disease in Korea. The incidence and clinical characteristics of Cushing's disease in Korea. J Kor Endocrinol Soc. 2000. 15:31–45.
10. Bochicchio D, Losa M, Buchfelder M. Factors influencing the immediate and late outcome of Cushing's disease treated by transsphenoidal surgery: a retrospective study by the European Cushing's Disease Survey Group. J Clin Endocrinol Metab. 1995. 80:3114–3120.

11. Gibson S, Ray DW, Crosby SR, Dornan TL, Jennings AM, Bevan JS, Davis JR, White A. Impaired processing of proopiomelanocortin in corticotroph macroadenomas. J Clin Endocrinol Metab. 1996. 81:497–502.

12. Pereira AM, van Aken MO, van Dulken H, Schutte PJ, Biermasz NR, Smit JW, Roelfsema F, Romijn JA. Long-term predictive value of postsurgical cortisol concentrations for cure and risk of recurrence in Cushing's disease. J Clin Endocrinol Metab. 2003. 88:5858–5864.

13. Hammer GD, Tyrrell JB, Lamborn KR, Applebury CB, Hannegan ET, Bell S, Rahl R, Lu A, Wilson CB. Transsphenoidal microsurgery for Cushing's disease: initial outcome and long-term results. J Clin Endocrinol Metab. 2004. 89:6348–6357.

14. Rees DA, Hanna FW, Davies JS, Mills RG, Vafidis J, Scanlon MF. Long-term follow-up results of transsphenoidal surgery for Cushing's disease in a single centre using strict criteria for remission. Clin Endocrinol (Oxf). 2002. 56:541–551.

15. Rollin GA, Ferreira NP, Junges M, Gross JL, Czepielewski MA. Dynamics of serum cortisol levels after transsphenoidal surgery in a cohort of patients with Cushing's disease. J Clin Endocrinol Metab. 2004. 89:1131–1139.

16. Esposito F, Dusick JR, Cohan P, Moftakhar P, McArthur D, Wang C, Swerdloff RS, Kelly DF. Clinical review: early morning cortisol levels as a predictor of remission after transsphenoidal surgery for Cushing's disease. J Clin Endocrinol Metab. 2006. 91:7–13.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download