Abstract
Bone scan (BS) and serum alkaline phosphatase (ALP) concentration are used to detect bone metastasis in malignancy, although whole-body fluoro-D-glucose positron emission tomography computed tomography (FDG PET/CT) is being used increasingly. But BS is still used for the detection of metastatic bone lesion. So we compared the usefulness of PET/CT, BS, and serum ALP in detecting bone metastases in patients with newly diagnosed lung cancer. The medical record database was queried to identify all patients with a new diagnosis of lung cancer between January 2004 and December 2005, who had a PET/CT, BS, and serum ALP before treatment. We retrospectively reviewed all patients' records and radiological reports. One hundred eighty-two patients met the inclusion criteria. Bone metastases were confirmed in 30 patients. The sensitivity values were 93.3% for PET/CT, 93.3% for BS, 26.7% for serum ALP concentration, and 26.7% for BS complemented with serum ALP concentration. The respective specificity values were 94.1%, 44.1%, 94.1%, and 97.3%. The kappa statistic suggested a poor agreement among the three modalities. FDG PET/CT and BS had similar sensitivity, but PET/CT had better specificity and accuracy than BS. PET/CT is more useful than BS for evaluating bone metastasis. However, in the advanced stage, because of its high specificity, BS complemented with serum ALP is a cost-effective modality to avoid having to use PET/CT.
Bone metastasis is present in 20-30% of patients at the initial diagnosis of lung cancer (1-3). Evaluating metastatic bone lesions is important in determining the therapeutic plan. Bone scan (BS) and serum alkaline phosphatase (ALP) concentration are used to diagnose bone metastasis in malignancy (4, 5). Although BS is sensitive in detecting advanced metastatic bone lesions, its specificity is less than optimal because infection, trauma, arthropathy, and neoplasm also may increase radionuclide uptake (6).
Whole-body fluoro-D-glucose positron emission tomography (FDG PET) and positron emission tomography computed tomography (PET/CT) are being used increasingly, especially for node staging (3). Several studies of PET and metastatic bone lesion have been reported (7, 8). PET is as accurate as BS in detecting metastatic bone lesions and has better specificity than BS, but there are few studies of bone metastases using PET/CT. The purpose of this study was to compare the usefulness of whole-body FDG PET/CT, BS, and serum ALP concentration in detecting bone metastases in patients with newly diagnosed lung cancer, and to find the role of BS in a new era of PET/CT.
The electronic medical record database at Seoul National University Hospital was reviewed retrospectively to identify all patients seen between January 2004 and December 2005 with a new diagnosis of lung cancer. Lung cancer in all patients was confirmed pathologically, and patients underwent both whole-body 2-deoxy-2-18F-fluoro-D-glucose (18F-FDG) PET/CT and BS for staging evaluation. Serum ALP concentration was measured within five days of the PET/CT scan in all patients. The PET/CT scan, BS, and measurement of ALP concentration were completed before initiation of therapy and after sufficient (six months or more) follow up. The study protocol was submitted to and approved by an internal review boards.
PET/CT studies were performed using a combined PET/CT scanner (Philips Gemini, DA best, Netherlands). PET/CT images were acquired 1 hr after injection of 0.14 mCi/kg of 18F-FDG in the 3 dimension mode, from the skull to the midthigh, at 7-9 bed positions of 2.5 min each. CT images were used for attenuation correction and fusion; no contrast medium was used. Helical CT was acquired first with the following parameters: 50 mAs, 120 kV, 5 mm section thickness, and 0.75 sec per CT rotation. The CT data were resized from a 512×512 matrix to a 144×144 matrix to match the PET/CT data to fuse the images.
BS was performed by the intravenous administration of technetium-99m methylene diphosphonate at a dose of 35-40 mCi. Images were obtained after 3 hr on a gamma camera (E.CAM, Siemens, Erlangen, Germany).
Two or three experienced nuclear medicine physicians interpreted the PET/CT studies and BS as positive for bone metastasis if they contained a focal increased uptake area.
Serum ALP concentration was measured with a Hitachi 747 autoanalyzer (Hitachi-Roche, Tokyo, Japan) using p-nitrophenyl phosphatase as the substrate. The reference range for serum ALP concentration was 30-115 IU/L in our institute. All values higher than the reference range were considered as positive for bone metastasis.
Bone metastases were confirmed using the following criteria: 1) progressing bone lesion on the follow-up scan (PET/CT or BS); 2) confirmed bone metastasis by simple radiography, CT, or magnetic resonance imaging (MRI); 3) positive initial findings on both BS and PET/CT in the same bone lesion with symptoms; and 4) histological confirmation. In a patient with clinical evidence of infection, trauma, or arthropathy, the results were classified as negative for bone metastasis even though scan results suggested the presence of metastasis. When the PET/CT and BS results were discordant, symptoms and additional results of plain radiography, CT, and MRI were considered. In patients with a positive PET/CT or BS, we reviewed the follow-up records six months or longer after the initial PET/CT scan, except for patients who had died. The average follow-up interval was 333 days. Patients who showed no evidence suggesting bone metastasis during the follow-up period were classified as having no bone metastasis.
The sensitivity, specificity, accuracy, positive predictive value, negative predictive value, and agreement between each modality were calculated. To evaluate the independent contributions of PET/CT, BS, and serum ALP concentration in predicting bone metastasis, the kappa (κ) statistic was calculated to determine the agreement between variables. The κ value was categorized as follows: poor (<0.30), good (0.31-0.60), and excellent (0.61-1.0). The detection of bone metastasis by PET/CT, BS, and serum ALP concentration were compared using the McNemar test; p<0.05 was considered significant. The mean serum ALP concentrations were compared between the 'with bone metastasis group' and the 'without bone metastasis group' using a t test. SPSS (version 12.0) and STATA (version 8.0) programs were used for statistical analysis.
We identified 182 patients eligible for analysis who met the inclusion criteria and who had sufficient follow-up periods. One hundred thirty-six were men, and 46 were women. The mean age of the patients was 61.8±10.4 yr. The average interval between PET/CT and BS was 8.5±14.0 days. The stage distribution and pathologic diagnosis of patients are given in Table 1. Forty-nine patients had stage 4 nonsmall cell lung cancer (NSCLC), and seven patients had extensive stage small cell lung cancer (SCLC).
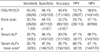
The PET/CT had 93.3% sensitivity, 94.1% specificity, 75.5% positive predictive value, 98.6% negative predictive value, and 93.4% accuracy (Table 4).
Thirty-seven patients had a positive PET/CT; 28 of these patients were confirmed with osseous metastasis.
patients were confirmed with osseous metastasis. Twenty six of 28 patients had bone metastasis with a positive bone scan finding. Bone metastasis was confirmed by MRI in 17 patients. Four patients had definite bone pain that could be explained only by bone metastasis. Three patients were confirmed by simple roentgenography, one patient by chest CT, and another patient by follow-up bone scan 131 days after the initial BS (Table 2).
Two patients of 28 patients had bone metastasis with a negative BS finding. One had left-side scapular pain (PET/CT-positive lesion) and a follow-up BS 102 days after the initial scan revealed progression of bone metastasis (its maximal standardised uptake value [SUV] was 3.2). Metastasis was confirmed in another patient as T-spine metastasis by spine MRI 122 days after the PET/CT scan (maximal SUV: 3.8).
Nine patients with a positive PET/CT finding were confirmed not to have bone metastasis.
Seven patients with positive PET/CT and bone scan findings were confirmed as having no bone metastasis by checking the MRI (one patient), follow-up BS (three patients), follow-up PET/CT (one patient), and definite trauma history (two patients).
Two patients with positive PET/CT and negative BS findings had no symptoms, and plain radiography did not identify any bony abnormality to suggest bone metastasis. In these patients, there was no evidence of bone metastasis during the follow up.
Two patients had positive results only in the BS (negative PET/CT results). One patient was confirmed to have bone metastasis by spine MRI at the initial staging evaluation. Another patient with positive BS findings on the spine had no symptoms, and this patient received a lobectomy (T2N0) without further evaluation. After the operation, the patient developed back pain, and simple roentgenography revealed bone metastasis progression.
The BS sensitivity was 93.5%, specificity was 44.1%, positive predictive value was 24.7%, negative predictive value was 97.1%, and accuracy was 52.2% (Table 4). The sensitivity of BS was equal to that of PET/CT. The false negative rates of PET/CT, and BS were identical at 6.7% in two patients for each modality. PET/CT missed 6.7% (two patients) of all bone metastasis patients, all of whom had a positive BS.
The mean value of serum ALP concentration was higher in patients with bone metastasis than in patients without bone metastasis. In lung cancer patients, the mean serum concentration of ALP was 103.3±60.0 IU/L in patients with bone metastasis and 78.5±23.1 IU/L in patients without bone metastasis (p=0.02). The BS complemented with serum alkaline phosphatase concentration sensitivity was 26.7%, specificity was 97.3%, positive predictive value was 66.7%, negative predictive value was 87.1%, and accuracy was 85.7% (Table 4).
The McNemar comparison test showed that the specificity and accuracy were significantly higher for PET/CT than for BS (p<0.001). The accuracy was also higher for PET/CT than for serum ALP concentration (p=0.002). BS complemented with serum ALP concentration gave no additional gain in sensitivity over PET/CT, but the specificity value was, at least, equal to that of PET/CT (p=0.26) (Table 3, 4).
The κ statistics were calculated for the three modalities. The κ value was 0.19 between PET/CT and bone scan, 0.03 between BS and serum ALP concentration, and 0.15 between serum ALP concentration and PET/CT. The low κ values suggested poor agreement between the three modalities (Table 5).
Studies of bone metastasis detection show that PET is significantly more accurate than BS in detecting various malignancies (e.g., prostate, breast, head and neck, and lung) (7-15).
We used FDG PET/CT instead of FDG PET. Several data from other studies suggest that PET/CT is more accurate in detecting tumors using 18F-FDG as the tracer than using 99m-Tc-polyphosphonates (16, 17). Several studies of bone metastasis have used PET with 18F-fluoride, a bone-imaging agent (nonspecific bone tracer). The uptake of the fluoride ion is two-fold higher than that of 99m-Tc-polyphosphonates (18). Schirrmeister and colleagues compared 99m-Tc-MDP BS and 18F-NaF PET in the diagnosis of bone metastasis (19). PET was more sensitive (91.6%) than BS (41.7%) in detecting both osteolytic and osteoblastic lesions. Even-Sapir and colleagues reported that 18F-fluoride PET/CT is both sensitive and specific in detecting lytic and sclerotic malignant lesions. In their study, the diagnostic accuracy was 88% for PET and 100% for PET/CT (p<0.05), and the specificity was 56% for PET and 88% for PET/CT (not significant) (9, 18). The authors of these studies attributed the better accuracy of PET/CT compared with PET to better spatial resolution because PET/CT allows the exclusion of many benign diseases, such as degenerative bone diseases, fractures, and inflammation, from the diagnosis of bone metastasis. In addition, some malignant lesions can be overlooked by PET; for example, some lytic bone metastasis can be detected only by PET/CT because of increased uptake at the margins of the lytic zone.
In a study of 110 patients with lung cancer, Bury and colleagues reported that FDG PET was as sensitive as, but had better specificity than, BS (8). Cheran and colleagues reported a significantly greater accuracy in FDG PET (95%) than in BS (90%) when equivocal BS results were excluded from the analysis, and reported a significantly greater sensitivity of PET (91%) than BS (63-75%) (7). We found a 93.3% sensitivity of PET/CT, which agrees with values of previous studies (Table 6). We found two patients with lesions with abnormal findings using PET/CT with normal BS finding. Lesions identified as abnormal in the BS and normal PET/CT findings were all spine lesions in our study (two patients). Because all lesions showed no increase in 18F-FDG uptake, we were unable to show that PET/CT was more effective than simple PET. In our study, 31% of patients had distant metastasis, but other studies found 39% and 40% of patients with distant metastasis (7, 8). The positive predictive value of PET/CT in our study was thus lower than that of PET in previous studies because previous studies enrolled more patients with bone metastases.
One study compared the detection of bone metastasis in 18F-fluoride and 18F-FDG PET (20). Both 18F-fluoride, as a nonspecific bone tracer, and 18F-FDG, as an agent to image altered tumor metabolism, have potential roles in the management of patients with skeletal metastasis, but no direct comparison has been reported. In a prospective study, Hoegerle and colleagues used a combined FDG and fluoride PET scan as an advanced metabolic imaging approach to evaluate cancer (21). Seventy-eight percent with positive 18F-fluoride PET findings and 88% with positive fluoride and FDG combination PET findings were confirmed with bone metastasis by morphologic imaging (CT, MRI). However, the improvement from 78% in 18F-fluoride PET-diagnosed patients to 88% in the combined study group was not significant. Considering that most studies involved FDG PET and node staging of lung cancer (3), bone metastasis evaluation using only 18F-fluoride PET is not preferable. More studies are needed to compare PET/CT and PET, especially when 18F-FDG is used as the tracer in diagnosing bone metastasis.
Ebert and colleagues reported 33.3% sensitivity and 97.5% specificity of ALP concentration in diagnosing bone metastasis (2). These results agree with our data. Although the accuracy was 83%, poor agreements among three diagnostic tools (FDG PET/CT, BS, and serum ALP) lowered the diagnostic value in our study. Other studies reported promising results for bone-specific ALP and newer bone markers (osteocalcin, urine deoxypyridinoline crosslinks, etc.), but serum total ALP concentration had a low diagnostic value for diagnosing metastatic bone disease (2, 22). Some studies have compared BS and serum ALP concentration in the detection of metastatic bone lesions (2, 23), although their complementary roles compared with PET/CT have not been studied. In our study, BS together with serum ALP concentration had a higher specificity than did PET/CT, although it was not significant (p=0.26).
In conclusion, although our data did not show a superior sensitivity of PET/CT over BS in the screening of metastatic bone lesions, PET/CT had higher specificity and accuracy. These data suggest that BS can be eliminated in staging work-up for preoperative patients who need PET/CT for nodal staging. However, in patients with disseminated disease who do not need evaluation of nodal staging, BS and the measurement of serum ALP concentration are sufficient for detecting asymptomatic metastatic bone lesions.
Figures and Tables
ACKNOWLEDGMENT
Special thanks to Chang-Hoon Lee for the use of STATA program and his advice about statistics.
References
1. Tritz DB, Doll DC, Ringenberg QS, Anderson S, Madsen R, Perry MC, Yarbro JW. Bone marrow involvement in small cell lung cancer. Clinical significance and correlation with routine laboratory variables. Cancer. 1989. 63:763–766.

2. Ebert W, Muley T, Herb KP, Schmidt-Gayk H. Comparison of bone scintigraphy with bone markers in the diagnosis of bone metastasis in lung carcinoma patients. Anticancer Res. 2004. 24:3193–3201.
3. Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest. 2003. 123:1 Suppl. 137S–146S.
4. Aruga A, Koizumi M, Hotta R, Takahashi S, Ogata E. Usefulness of bone metabolic markers in the diagnosis and follow-up of bone metastasis from lung cancer. Br J Cancer. 1997. 76:760–764.

5. Chung JH, Park MS, Kim YS, Chang J, Kim JH, Kim SK, Kim SK. Usefulness of bone metabolic markers in the diagnosis of bone metastasis from lung cancer. Yonsei Med J. 2005. 46:388–393.

6. Peterson JJ, Kransdorf MJ, O'Connor MI. Diagnosis of occult bone metastases: positron emission tomography. Clin Orthop Relat Res. 2003. S120–S128.

7. Cheran SK, Herndon JE 2nd, Patz EF Jr. Comparison of whole-body FDG-PET to bone scan for detection of bone metastases in patients with a new diagnosis of lung cancer. Lung Cancer. 2004. 44:317–325.

8. Bury T, Barreto A, Daenen F, Barthelemy N, Ghaye B, Rigo P. Fluorine-18 deoxyglucose positron emission tomography for the detection of bone metastases in patients with non-small cell lung cancer. Eur J Nucl Med. 1998. 25:1244–1247.

9. Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-Fluoride PET, and 18F-Fluoride PET/CT. J Nucl Med. 2006. 47:287–297.
10. Shreve PD, Grossman HB, Gross MD, Wahl RL. Metastatic prostate cancer: initial findings of PET with 2-deoxy-2-[F-18]fluoro-D-glucose. Radiology. 1996. 199:751–756.

11. Abe K, Sasaki M, Kuwabara Y, Koga H, Baba S, Hayashi K, Takahashi N, Honda H. Comparison of 18FDG-PET with 99mTc-HMDP scintigraphy for the detection of bone metastases in patients with breast cancer. Ann Nucl Med. 2005. 19:573–579.
12. Liu FY, Chang JT, Wang HM, Liao CT, Kang CJ, Ng SK, Chan SC, Yen TC. [18F]fluorodeoxyglucose positron emission tomography is more sensitive than skeletal scintigraphy for detecting bone metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol. 2006. 24:599–604.

13. Yang SN, Liang JA, Lin FJ, Kao CH, Lin CC, Lee CC. Comparing whole body (18)F-2-deoxyglucose positron emission tomography and technetium-99m methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J Cancer Res Clin Oncol. 2002. 128:325–328.
14. Ohta M, Tokuda Y, Suzuki Y, Kubota M, Makuuchi H, Tajima T, Nasu S, Suzuki Y, Yasuda S, Shohtsu A. Whole body PET for the evaluation of bony metastases in patients with breast cancer: comparison with 99Tcm-MDP bone scintigraphy. Nucl Med Commun. 2001. 22:875–879.

15. Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998. 16:3375–3379.

16. Hany TF, Steinert HC, Goerres GW, Buck A, von Schulthess GK. PET diagnostic accuracy: improvement with in-line PET-CT system: initial results. Radiology. 2002. 225:575–581.

17. Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A, Altman H, Keidar Z, Israel O. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med. 2003. 44:1200–1209.
18. Even-Sapir E, Metser U, Flusser G, Zuriel L, Kollender Y, Lerman H, Lievshitz G, Ron I, Mishani E. Assessment of malignant skeletal disease: initial experience with 18F-fluoride PET/CT and comparison between 18F-fluoride PET and 18F-fluoride PET/CT. J Nucl Med. 2004. 45:272–278.
19. Schirrmeister H, Glatting G, Hetzel J, Nussle K, Arslandemir C, Buck AK, Dziuk K, Gabelmann A, Reske SN, Hetzel M. Prospective evaluation of the clinical value of planar bone scans, SPECT, and (18)F-labeled NaF PET in newly diagnosed lung cancer. J Nucl Med. 2001. 42:1800–1804.
20. Cook GJ, Fogelman I. Detection of bone metastases in cancer patients by 18F-fluoride and 18F-fluorodeoxyglucose positron emission tomography. Q J Nucl Med. 2001. 45:47–52.
21. Hoegerle S, Juengling F, Otte A, Altehoefer C, Moser EA, Nitzsche EU. Combined FDG and [F-18]fluoride whole-body PET: a feasible two-in-one approach to cancer imaging? Radiology. 1998. 209:253–258.

22. Alatas F, Alatas O, Metintas M, Colak O, Erginel S, Harmanci E. Usefulness of bone markers for detection of bone metastases in lung cancer patients. Clin Biochem. 2002. 35:293–296.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download