Abstract
Dystrophic epidermolysis bullosa (DEB) are caused by mutations in the COL7A1 gene, which encodes type VII collagen. Even though more than 500 different COL7A1 mutations have been identified in DEB, it still remains to be under-investigated. To investigate the mutation of COL7A1 in moderately severe phenotype of recessive DEB (RDEB) in a Korean patient, the mutation detection strategy was consisted of polymerase chain reaction (PCR) amplification of genomic DNA, followed by heteroduplex analysis, nucleotide sequencing of the PCR products demonstrating altered mobility. In this study, we found that one mutation (c.8569G>T) was detected within exon 116. The mutation of c.8569G>T in exon 116 changed the GAG (Glu) to TAG, eventually resulted in premature termination of type VII collagen polypeptide. Furthermore the mother did not have the mutation c.8569G>T in exon 116. The other novel mutation (c.4879G>A) was detected within exon 51 of both patient and mother, thereby resulting in changing valine (Val) to isoleucine (Ile) in type VII collagen polypeptide. Taken together, in this study we identified compound heterozygosity for COL7A1 mutations (c.8569G>T and c.4879G>A) in moderately severe RDEB in a Korean patient. We hope that this data contribute to the expanding database on COL7A1 mutations in DEB.
Dystrophic epidermolysis bullosa (DEB) is caused by mutations in the COL7A1 gene encoding collagen VII, the major component of anchoring fibrils (1, 2). DEB is clinically characterized by mucocutaneous blistering in response to minor trauma, followed by scarring and nail dystrophy. It occurs as either an autosomal dominant (DDEB) or recessive (RDEB) trait, each form having unique clinical features and severity (3).
The most severe RDEB subtype, the Hallopeau-Simens (HS) type, shows a complete lack of expression of type VII collagen, whereas some collagen expression is found in the non-Hallopeau-Siemens (non-HS) type (4, 5). The most severe form, HS-RDEB, was usually caused by combinations of premature termination codon (PTC) mutations (6, 7). The non-HS type with the presence of some type VII collagen expression is generally due to a combination of PTC with mis-sense mutations including glycine substitution, in-frame deletion or a splice site mutation (8). These glycine substitution mutations cause various phenotypes. In the heterozygous state, some glycine substitution mutations cause a dominant DEB phenotype through a dominant negative interference or cause limited nail dystrophy or no phenotype at all (9-11). However, glycine substitution mutations lead to a moderately severe phenotype of RDEB when in the homozygous state or when combined with a PTC in the other COL7A1 allele (12).
In this study, we have systematically screened the COL7A1 gene, for mutational analysis in a RDEB Korean patient, 15-yr-old male, showing multiple erosion, crusts, milia formation on scar, mitten-like deformity of hand, and dysphagia, due to esophageal stricture, evidenced by esphagogram examination.
A 15-yr-old male patient visited, suffering from multiple erosion, deformity of hand, and dysphagia. Several trauma-induced erosions and blister had developed mainly on his hands and trunk since infancy. His parents were non-consanguineous, and his family members including his siblings were clinically unaffected.
For amplification of segments of genomic DNA, the primers were designed on the basis of flanking intronic sequences as previously reported by Christiano et al. (13). The oligomer primers were 20 nucleotides in length, contained no greater than three identical consecutive bases, minimal secondary structure, between 45 and 65% G/C content, and were free of the potential for primer-dimer formation. Amplimers ranged from 196 to 589 bp in size. Primers were synthesized using an automated oligonucleotide synthesizer (Bioneer Co., Daejeon, Korea).
For PCR amplification of genomic sequences, DNA was isolated from peripheral blood by the DNA extraction kit (Qiagen Co., Valencia, CA, U.S.A.), according to the manufacturer's recommendation. Purified DNA was used as a template in a reaction volume of 50 µL, containing 20 pM of each primer, 100 nM MgCl2, 20 mM of each nucleotide, and 2.5 U Taq polymerase (Perkin-Elmer, Cetus, MO, U.S.A.). The conditions for PCR were similar for each primer pair, except that the annealing temperature varied. After denaturation for 5 min at 94℃, each PCR cycle consisted of 94℃, 45 sec; annealing 55-61℃, 45 sec; 72℃ 45 sec; for 40 cycles. The PCR amplification products were examined on 2% agarose gels. The PCR amplimers were examined for detection of sequence variants by electrophoretic scanning techniques such as the heterodulpex analyses described below.
Heteroduplex analyses were performed using conformation-sensitive gel electrophoresis (CSGE), as described by Ganguly et al. (14). For CSGE, 8 µL of each sample was denatured by heating at 98℃ for 5 min, followed by cooling at 68℃ for 1 hr. Loading dye (2 µL) was added and the samples were run on the CSGE gel overnight at 300 V in 0.25×TBE buffer (top chamber) and 1×TBE buffer (bottom chamber). Visualization of bands was then performed in ethidium bromide solution (1 ng/mL in 0.5×TBE).
If bands with altered mobility were detected, the PCR products were sequenced. In some cases, the PCR products were subcloned into the TA-cloning vector according to the manufacturer's recommendation (Invitrogen, San Diego, CA, U.S.A.). Plasmid minipreparations were performed using the standard boiling methods, and several positive clones were sequenced by the dideoxynucleotide sequencing method (15). Alternatively, the PCR products were purified on a chromatographic column (QIAquick Spin column, Qiagen, Valencia, CA) and then subjected to direct dideoxynucleotide sequencing in an automated DNA sequencer (Applied Biosystems, Foster, CA, U.S.A.) with the same primers used for PCR amplification. The variant sequences in patients affected with DEB were compared with those in unaffected family members. Comparative sequence analysis with the normal COL7A1 template sequence, deposited in Gene Bank under accession No. L23982, was also performed.
The patient showed the multiple erosion and crusts formation on trunk with scattered blister formation (Fig. 1A, B). He also was suffered from dysphagia due to stricture of gastroesophageal junction, observed by esophagography. His fingers and toes were shortened and contracted due to repeated blister formation, healing, and eventually scarring with mild fusion between webs (Fig. 1C, D). The pedigree showed that there is no affected person's among parents and relatives, except the patient (Fig. 2).
Heteroduplex analyses using amplified DNA from RDEB family revealed band shifts in the affected individual for PCR products spanning, within exon 116. The homoduplex band was seen in his mother. Unfortunately, we did not perform heteroduplex analysis of DNA from his father, due to the divorce state. Direct sequencing of the PCR products from the patient revealed G to T transition at position 8569. As a result, the codon for glutamic acid (GAG) is changed to a codon for stop (TAG), and this mutation was designated c.8569G>T and did not present in the maternal DNA samples. (Fig. 4A). Furthermore the mutation of c.8569G>T was confirmed by restriction enzyme (XspI), specifically recognizes and digests in CTAG nucleotide sequence. A further study revealed that G to A transition at position 4879 within exon 51. As a result, the codon for valine (VAL) is changed to a codon for isoleucine (Ile), and this mutation was detected both patient and mother DNA samples. Thus, our patient has a premature termination codon (Glu → Stop, 2857) mutation in one allele and missense mutation (Val → Ile, 1627) in the other allele. The mother and father may be carrier state and remain unaffected clinically (Fig. 5).
EB is a group of inherited skin diseases characterized by blistering and scarring of the skin after mild trauma. DEB is transmitted in an autosomal dominant or recessive pattern, both forms showing tissue separation beneath the basement membrane at the level of the anchoring fibrils (16). Type VII collagen molecules, major component of anchoring fibrils, are homotrimers, each pro α1 (VII) polypeptide chain containing a central triple-helical collagenous domain (145 kDa in size) flanked by both a large (145 kDa) amino-terminal noncollagenous (NC-1) domain and a small (30 kDa) carboxy-terminal noncollagenaous (NC-2) domain (17). The NC-1 domain consists of submodules with homology to an adhesive protein, including a matrix-protein motif, a fibronectin like domain, and a von Willebrand factor A domain (18). The NC-2 domain contains conserved cysteins involved in the formation of disulfide linkage between type VII collagen homotrimers (19, 20). In the conversion of procollagen VII to collagen VII, two procollagen VII molecules form an anti-parallel dimmer with eight cysteines in the carboxyl-terminal NC-2 domain, which is finally removed at the procollagen C-proteinase cleavage site. RDEB comprises several forms, the most severe of which is the Hallopeau-Siemens type (HS-RDEB). Other forms of RDEB comprise the mitis (M-RDEB), the inverse, and the localized types. It has been suggested that the nature and/or the location of mutations in COL7A1 determine the variations in clinical phenotype and type VII collagen expression. PTC mutations are silent when in a heterozygous state, but when homozygous or combined with another PTC mutation in other alleles, the consequences are severe in terms of skin fragility, as illustrated by HS-RDEB (9-12). In support of this interpretation, a majority of mutations reported in HS-RDEB result from PTC, which are thought to lead to null alleles through PTC-mediated mRNA decay (21). PTC association with either a glycine substitution, a slice-site mutation, or, a delayed termination codon have also been characterized in clinically moderate form of RDEB (12, 22, 23).
It has been known that Hallopeau-Siemens variant RDEB involves PTC mutations on both COL7A1 alleles leading to an absence of collagen VII and no detectable anchoring fibrils. However, in this study, electron microscopy examination provided evidence that the synthesis of rudimentary anchoring fibrils was still possible although its amount was considerably reduced. We hypothesized that some anchoring fibrils were formed in spite of the PTC mutations on COL7A1 allele, and maybe that is why the skin fragility and scar formation was not so severe in our patient. Actually, our patient showed a PTC (c.8569G>T) in exon 116. Exon 116 is located in the middle of the NC-2 domain, and we hypothesized that the truncated procollagen VII from the mutant allele with a PTC in exon 116 included two out of eight cysteines needed for disulfide bond formation, and hence a few functional anchoring fibrils could be formed. It is possible that the mutant allele of c.8569G>T may be inherited from father, due to the mother allele is normal. However, it still remains to be determined this mutant allele is really inherited from father. The both patient and mother have another novel mutation that G to A transition at position 4879 within exon 51; the codon for valine (VAL) is changed to a codon for isoleucine (Ile), and resulting in the substitution is occurred within the collagenous domain that form the collagen VII triple helix. In this study, the mother did not show any signs of mucocutaneous and nail involvement despite a careful reexamination. It still needs to investigate the maternal mutation c.4879G>A will be interpreted as a silent mutation or altered splicing.
In summary, in this study, we reported a moderately severe RDEB patient, who has the compound mutation of c.8569G>T in exon 116 and c.4879G>A within exon 51. We hope that this data contribute to the expanding database on COL7A1 mutations in DEB, and accumulating mutational data on patients with RDEB have been proved useful in providing more precise clinical information, particularly in defining diagnosis and in improving genetic counseling.
Figures and Tables
Fig. 1
Clinical features. Blisters and erosions were seen on the trunk (A, B) as well as milia (inset). Acral scaring and loss of finger and toe nail (C, D).
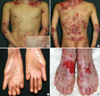
Fig. 2
The schematic presentation of the pedigree of the patient. The black arrow indicates the patient.
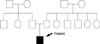
Fig. 3
Histological findings. Subepidermal blister formation and flattened rete ridges are observed (A) (H&E, ×200). Electron micrograph showing a few amount of anchoring fibrils (arrow heads) beneath the lamina densa (LD) (B).
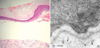
Fig. 4
Identification of the mutations in COL7A1 in patient. Direct sequencing of the patient PCR product revealed G to T transition at position 8569 (A). As a result, the codon for Glu (GAG) is changed to a codon for stop (TAG). This mutation was confirmed by restriction enzyme, XspI (C). Schematic representation of the domain structure of procollagen VII and the location of the premature stop codon (E2857X) in exon 116 (D). Cysteine residues are marked as a "C", and an asterisk (*) shows the procollagen C-proteinase cleavage site.

Fig. 5
Identification of the mutations in COL7A1 in the patient and mother. Direct sequencing of the patient PCR product revealed G to A transition at position 4879 (A). As a result, the codon for Val (GUU) is changed to a codon for Ile (AUU). This mutation was confirmed by restriction enzyme, Alw NI (B).

References
1. Uitto J, Pulkkinen L, Christiano AM. Molecular basis of the dystrophic and junctional forms of epidermolysis bullosa: mutations in the type VII collagen and kalinin (laminin 5) genes. J Invest Dermatol. 1994. 103:5 Suppl. 39S–46S.

2. Varki R, Sadowski S, Uitto J, Pfendner E. Epidermolysis bullosa. II. Type VII collagen mutations and phenotype-genotype correlations in the dystrophic subtypes. J Med Genet. 2007. 44:181–192.

3. Hamada T, Fukuda S, Ishii N, Sakaguchi S, Ishikawa T, Abe T, Yasumoto S, Hashimoto T, Nakano H, Sawamura D. Genotype-phenotype correlation in non-Hallopeau-Siemens recessive dystrophic epidermolysis bullosa: the splice site mutation c.6216+5G>T in the COL7A1 gene results in aberrant and normal splicings. J Dermatol Sci. 2008. 50:147–150.
4. Uitto J, Hovnanian A, Christiano AM. Premature termination codon mutations in the type VII collagen gene (COL7A1) underlie severe recessive dystrophic epidermolysis bullosa. Proc Assoc Am Physicians. 1995. 107:245–252.
5. Christiano AM, Amano S, Eichenfield LF, Burgeson RE, Uitto J. Premature termination codon mutations in the type VII collagen gene in recessive dystrophic epidermolysis bullosa result in nonsense-mediated mRNA decay and absence of functional protein. J Invest Dermatol. 1997. 109:390–394.

6. Hovnanian A, Rochat A, Bodemer C, Petit E, Rivers CA, Prost C, Fraitag S, Christiano AM, Uitto J, Lathrop M, Barrandon Y, de Prost Y. Characterization of 18 new mutations in COL7A1 in recessive dystrophic epidermolysis bullosa provides evidence for distinct molecular mechanisms underlying defective anchoring fibril formation. Am J Hum Genet. 1997. 61:599–610.
7. Oh SW, Lee JS, Kim MY, Kim SC. COL7A1 mutational analysis in Korean patients with dystrophic epidermolysis bullosa. Br J Dermatol. 2007. 157:1260–1264.
8. Christiano AM, McGrath JA, Tan KC, Uitto J. Glycine substitutions in the triple-helical region of type VII collagen result in a spectrum of dystrophic epidermolysis bullosa phenotypes and patterns of inheritance. Am J Hum Genet. 1996. 58:671–681.
9. Terracina M, Posteraro P, Schubert M, Sonego G, Atzori F, Zambruno G, Bruckner-Tuderman L, Castiglia D. Compound heterozygosity for a recessive glycine substitution and a splice site mutation in the COL7A1 gene causes an unusually mild form of localized recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1998. 111:744–750.
10. Shimizu H, Hammami-Hauasli N, Hatta N, Nishikawa T, Bruckner-Tuderman L. Compound heterozygosity for silent and dominant glycine substitution mutations in COL7A1 leads to a marked transient intracytoplasmic retention of procollagen VII and a moderately severe dystrophic epidermolysis bullosa phenotype. J Invest Dermatol. 1999. 113:419–421.
11. Kim J, Kim SC, Yasukawa K, Shimizu H. Compound heterozygosity for premature termination codon and glycine substitution mutations in the COL7A1 gene in Korean siblings with a moderately severe phenotype of recessive dystrophic epidermolysis bullosa. J Dermatol Sci. 2003. 33:180–183.
12. Masunaga T, Shimizu H, Takizawa Y, Uitto J, Nishikawa T. Combination of novel premature termination codon and glycine substitution mutations in COL7A1 leads to moderately severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2000. 114:204–205.
13. Christiano AM, Hoffman GG, Zhang X, Xu Y, Tamai Y, Greenspan DS, Uitto J. Strategy for identification of sequence variants in COL7A1 and a novel 2-bp deletion mutation in recessive dystrophic epidermolysis bullosa. Hum Mutat. 1997. 10:408–414.
14. Ganguly A, Rock MJ, Prockop DJ. Conformation-sensitive gel electrophoresis for rapid detection of single-base differences in double-stranded PCR products and DNA fragments: evidence for solvent-induced bends in DNA heteroduplexes. Proc Natl Acad Sci USA. 1993. 90:10325–10329.

15. Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977. 74:5463–5467.

16. Fine JD, McGrath J, Eady RA. Inherited epidermolysis bullosa comes into the new millenium: a revised classification system based on current knowledge of pathogenetic mechanisms and the clinical, laboratory, and epidemiologic findings of large, well-defined patient cohorts. J Am Acad Dermatol. 2000. 43:135–137.

17. Christiano AM, Greenspan DS, Lee S, Uitto J. Cloning of human type VII collagen. Complete primary sequence of the alpha 1(VII) chain and identification of intragenic polymorphisms. J Biol Chem. 1994. 269:20256–20262.

18. Christiano AM, Rosenbaum LM, Chung-Honet LC, Parente MG, Woodley DT, Pan TC, Zhang RZ, Chu ML, Burgeson RE, Uitto J. The large non-collagenous domain (NC-1) of type VII collagen is amino-terminal and chimeric. Homology to cartilage matrix protein, the type III domains of fibronectin and the A domains of von Willebrand factor. Hum Mol Genet. 1992. 1:475–481.

19. Hammami-Hauasli N, Schumann H, Raghunath M, Kilgus O, Luthi U, Luger T, Bruckner-Tuderman L. Some, but not all, glycine substitution mutations in COL7A1 result in intracellular accumulation of collagen VII, loss of anchoring fibrils, and skin blistering. J Biol Chem. 1998. 273:19228–19234.
20. Burgeson RE. Type VII collagen, anchoring fibrils, and epidermolysis bullosa. J Invest Dermatol. 1993. 101:252–255.

21. Christiano AM, McGrath JA, Uitto J. Influence of the second COL7A1 mutation in determining the phenotypic severity of recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1996. 106:766–770.
22. Matsunaga A, Sasaki J, Han H, Huang W, Kugi M, Koga T, Ichiki S, Shinkawa T, Arakawa K. Compound heterozygosity for an apolipoprotein A1 gene promoter mutation and a structural nonsense mutation with apolipoprotein A1 deficiency. Arterioscler Thromb Vasc Biol. 1999. 19:348–355.

23. Tamai K, Ishida-Yamamoto A, Matsuo S, Iizuka H, Hashimoto I, Christiano AM, Uitto J, McGrath JA. Compound heterozygosity for a nonsense mutation and a splice site mutation in the type VII collagen gene (COL7A1) in recessive dystrophic epidermolysis bullosa. Lab Invest. 1997. 76:209–217.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download