Abstract
The intensity-modulated radiation therapy (IMRT) planning strategies for nasopharyngeal cancer among Korean radiation oncology facilities were investigated. Five institutions with IMRT planning capacity using the same planning system were invited to participate in this study. The institutions were requested to produce the best plan possible for 2 cases that would deliver 70 Gy to the planning target volume of gross tumor (PTV1), 59.4 Gy to the PTV2, and 51.5 Gy to the PTV3 in which elective irradiation was required. The advised fractionation number was 33. The planning parameters, resultant dose distributions, and biological indices were compared. We found 2-3-fold variations in the volume of treatment targets. Similar degree of variation was found in the delineation of normal tissue. The physician-related factors in IMRT planning had more influence on the plan quality. The inhomogeneity index of PTV dose ranged from 4 to 49% in Case 1, and from 5 to 46% in Case 2. Variation in tumor control probabilities for the primary lesion and involved LNs was less marked. Normal tissue complication probabilities for parotid glands and skin showed marked variation. Results from this study suggest that greater efforts in providing training and continuing education in terms of IMRT planning parameters usually set by physician are necessary for the successful implementation of IMRT.
Intensity modulated radiation therapy (IMRT) is a new approach to the planning and delivery of radiation (1, 2). Unlike 2-dimensional radiotherapy (2D-RT) and 3-dimensional radiotherapy (3D-CRT), IMRT allows more conformal dose coverage of the clinical target volume (CTV) in three dimensions, thereby sparing the surrounding normal tissues. Highly conformal treatment plans may reduce the risk of radiation toxicities and provide a means of potentially escalating the dose to target in selected patients, thus improving tumor control.
Head and neck cancers, especially nasopharyngeal cancer, are good candidates for IMRT because of their horseshoe-shaped CTVs and many critical normal organs that surround the CTV (3). Improving the conformity of the radiation dose to targets in the head and neck using IMRT promises reduced toxicity and, in some cases, improved loco-regional tumor control (3-7). Recently published phase III studies confirmed that IMRT provides superior treatment results with lower morbidity than conventional 2D- or 3D-CRT (8, 9).
IMRT may actually be disadvantageous in some situations, however, because it is relatively difficult to plan and administer. The planning process for IMRT is greatly influenced by physician-dependent factors such as segmentation of the target volume and non-tumor tissues on the planning CT, and specifying the dose to the target and the surrounding normal organs. Although many hospitals are now equipped with IMRT capabilities, the excellence of next level practice achieved by the use of IMRT could differ among hospitals depending on the clinical situation and the treatment team. Preparedness in performing IMRT is a crucial factor in its implementation.
In Korea, IMRT practices were established in several centers in 2001, and it is now being rapidly adopted. According to a 2006 survey conducted by the Korean Society for Therapeutic Radiology and Oncology (KOSTRO), 22 of 61 (35%) radiotherapy facilities nationwide possessed the hardware capacity necessary to implement IMRT practice (10). Although several Korean facilities have undertaken planning studies and explored the feasibility of IMRT in their clinic, it appears that a lack of preparedness exists in terms of fully implementing IMRT as a clinical routine (3, 4, 11-13).
The purpose of the present study was to investigate IMRT planning strategies for nasopharyngeal cancer among Korean radiation oncology facilities. We compared the planning parameters, resultant dose distributions, and values of biological indices, including normal tissue complication probabilities (NTCP) and tumor control probabilities (TCP), with a treatment plan generated in five different institutions using the same radiation treatment planning system (RTPS) for the same clinical cases. We also discussed the measures needed to improve the degree of clinical excellence in implementing IMRT as an advanced treatment technology.
In May 2006, five institutions, all with the same RTPS with IMRT planning capacity, were invited to participate in this study. As planning images, contrast-enhanced computed tomography (CT) scans were provided for Case 1. For Case 2, both contrast-enhanced CT scans and positron emission tomography (PET) images were provided. Planning images for the study cases were obtained on a Discovery ST PET-CT scanner (GE Healthcare, Milwaukee, WI, U.S.A.), using a slice thickness of 0.37 cm, and with the patients immobilized in the supine position using a Type S thermoplastic mask (Medtec, Orange City, IA, U.S.A.). Planning images were de-identified prior to use in the study. The employed IMRT planning platform was the p3-IMRT inverse planning module of Pinnacle3 (Philips, Fitchburg, WI, U.S.A.) commercial RTPS. Back-up CD disks were prepared with the treatment plans for the study cases; these plans had no added regions of interests (ROIs), beams, or inverse planning parameters. The beam commissioning data for a standard linear accelerator was added to these plans and used for dose calculations. We used the beam data of a Primus linear accelerator (Siemens, Munich, Germany) housed at a participating institution.
Each institution received the back-up CDs. The plans for the two clinical cases were restored in the RTPS of each institution. IMRT plans were performed in each institution according to the study guidelines, up to the step of generating ideal intensity maps for the beams. The treatment plans from each institution were then compiled and restored in a planning computer as different trials of the treatment planning data; the data were then analyzed.
The participating institutions were requested to produce the best plan possible that would deliver 70 Gy to the planning target volume of gross tumor (PTV1), 59.4 Gy to the region of high-risk regional lymph nodes (PTV2), and 51.5 Gy to the lymph node area (PTV3) in which elective irradiation was required. The advised fractionation number was 33. Delineation of the target volume and normal organs, the number and orientations of the beams, and the prescribed number and type of dose constraints were left to the discretion of the planning teams in each institution.
Case 1 was a 50-yr-old male diagnosed with cT2aN1 poorly differentiated squamous cell carcinoma of the nasopharynx. The primary tumor involved the right side of the Rosenmüller fossa and posterior wall of the nasopharynx, and extended to the posterior oropharyngeal wall. There was lymph node (LN) metastasis to the right level 2 area.
Case 2 was a 24-yr-old female diagnosed with T2bN2 undifferentiated carcinoma of the nasopharynx. The primary tumor involved the left side of the nasopharynx and extended to the parapharyngeal fatty tissues. There was tumor involvement in the left-sided retropharyngeal LNs and bilateral level 2 LNs.
IMRT planning strategies were compared. Parameters compared in the planning process included the delineation of target volume and normal organs, the number and orientations of the beams, and the prescribed number and type of dose constraints for optimization of the inverse planning.
General features of the planning results were compared, including the prescribed monitor unit (MU), maximum dose and its location, inhomogeneity index of the dose distribution to individual institution's PTV, and the deviation of D50 from the planning goal. The homogeneity index was defined as (D5-D95)/(Dmean). Dvolume was defined as the dose level where the cumulative dose volume histogram (DVH) intersects with the given volume of the ROI. Dmean was defined as the mean dose received by the ROI. The distributions of the isodose curve and DVHs for individual institution's PTV were also compared.
The ROIs delineated by the institution that provided the clinical case were set as the standard ROIs. To avoid potential difficulties in comparing plans that might have risen had ROIs been delineated separately by individual institutions, both standard ROIs and individual institution's ROIs were used for the comparison of dose statistics and biological indices among institutions. TCP was calculated via the Okunieff model, using the values of the dose of 50% tumor control (TCD50), slope50, and γ50 (14). NTCP was calculated via the Lyman-Kutcher-Burman model, using the Kutcher-Burman histogram reduction scheme, with n and m as determined by Burmann et al. (15, 16).
We found differences in the volumes contoured as the gross tumor volume (GTV) of the primary tumor and the involved LNs (Table 1). GTV of the primary tumor was 13.8-28.5 cm3 in Case 1, and 35.8-69.6 cm3 in Case 2. The volumes of PTV1, PTV2, and PTV3 were also different: we found 2-fold variation in the volume of PTV1, and a 3-fold variation in the volume of PTV2. One institution did not delineate PTV3 in Case 1, and three institutions did not delineate PTV3 in Case 2. This marked variation was due to difference in the level of lymph node areas treated as high risk area, and those treated as areas for elective nodal irradiation (Table 2). The major difference among institutions consisted of whether they decided to treat the level 1 and contralateral level 5 lymph node areas.
The variations encountered in contouring of the target volumes are presented on the same axial CT slice (Fig. 1). The contoured GTV for Case 2 was more consistent than that of Case 1. Although the degree of volumetric differences was the same in both cases, delineation of the GTV assisted by PET images appeared to cover the gross tumor more consistently, as in Case 2. For delineation of PTV2 and PTV3, almost all of the institutions used geometrical extension from the margin of anatomical structures such as regional LN areas; however, in constructing PTV2, Institution 2 used the direct geometrical extension from PTV1 without consideration for the anatomical margin of the regional LN area.
A similar degree of variation was identified in the delineation of normal tissue. All institutions delineated the parotid glands, eye balls, lens, optic nerve, and spinal cord. Not all institutions delineated the brainstem, pituitary gland, optic chiasm, oral cavity, trachea, esophagus, inner ear, vocal cord, brain, and temporal lobe of the brain. Some institutions excluded the deep lobe of the parotid gland from the structures to be spared, especially in the involved side (Fig. 2). Only two institutions used the planning at risk volume (PRV), which is expanded with margins ranging from 2 to 3 mm from contoured normal tissues such as lens, brainstem, and spinal cord.
Wide variation also existed in the setting of planning parameters (Table 4). Only one institution used the split-field IMRT technique, in which the low anterior neck is treated with an anterior field and matched with the IMRT portion. All of the others used extended whole-field IMRT, in which all of the target volumes are included within the IMRT field. The number of employed beams ranged from 7 to 11. All institutions used coplanar beams with equally spaced angles.
The p3-IMRT inverse planning module of Pinnacle3 provides two different methods for prescribing constraints for optimization. A hard constraint is the maximum or minimum dose for a ROI to be met absolutely while optimizing beam intensity, whereas a DVH constraint is the dose prescribed to a certain percentage of ROI volume that can be bargained using a weighting factor for each constraint. Only one institution applied hard constraints. The number of prescribed constraints ranged from 7 to 29, with a variety of weighting factors applied for each constraint.
The general features of the planning results are presented in Table 5. The number of MUs planned to be delivered showed wide variability: 466-1,134 in Case 1 and 490-2,269 in Case 2. MU was normalized for 90% of PTV1 to be covered with 70 Gy in Institution 2, and for 50% in Institution 5. The other institutions did not normalize MU for target coverage. The maximum dose ranged from 72.7 to 90.1 Gy in Case 1 and from 74.7 to 167.8 Gy in Case 2. The skin was the location of maximum dose in half of the plans assessed. The inhomogeneity index of PTV dose ranged from 4 to 49% in Case 1, and from 5 to 46% in Case 2. The percentage deviation of D50 from the dose of the planning goal was greater in PTV2 and PTV3 than in PTV1.
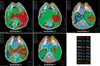
Because the individual institution's ROIs and the employed planning parameters were different for each institution, there was a striking range in the distribution of isodose curves and the DVH (Fig. 4, 5). The resulting plans by Institutions 1 and 2 showed excess dose deposit in the skin, oral cavity, and the soft tissues in the neck. The inferior border of the retropharyngeal lymph node was insufficiently covered in Institution 5. DVH for PTV2 showed that some institutions failed to obtain a sharp dose fall-off at the border between PTV1 and PTV2, and that the high-dose area in PTV2 was even hotter than that in PTV1 in one institution.
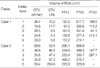
For given clinical cases, the dose statistics showing IMRT dose delivery patterns showed marked variations for both of standard ROIs and individual institution's ROIs among institutions (Table 6). The values of D95 for PTV1 were less than the prescribed dose in all plans. In Institution 1, D5 for PTV1 was more than 110% of the prescribed dose. The sparing of the parotid glands was tightest in Institution 5, with consideration that Institution 5 delineated the deep lobe on both sides. The other institutions tried to spare at least one parotid gland, located at the contralateral side of the primary tumor. D5 values for the spinal cord exceeded 45 Gy in one institution for standard ROI and in two institutions for individual institution's ROI. The value of D5 for brainstem exceeded 54 Gy in one institution.
In contrast to the profound variations recorded in dose statistics for PTV, variation in TCP for the primary lesion and involved lymph nodes was not so pronounced for both standard ROIs and individual institution's ROIs (Table 7). The NTCP for parotid glands and skin was largely unsatisfactory, showing marked variation among institutions. Considering the trend to sacrifice the parotid gland of the involved side, the NTCP for the contralateral side still showed unsatisfactory results in some institutions. Excess dose delivery to the skin, as shown in Fig. 4, explains the high NTCP of the skin in Institutions 1 and 2.
In Korea, IMRT is in the early stage of implementation, with routine use limited to a small number of hospitals; however, more hospitals are being equipped with modern state-of-the-art IMRT technology (10). Some institutions in Korea have performed the planning studies and explored the feasibility of IMRT in the clinical settings (3, 4, 11-13). The Korean Radiation Oncology Group (KROG) is currently performing a study regarding the optimal radiation prescription using IMRT in applying simultaneous integrated boost for nasopharyngeal cancer (KROG-0501).
Early publications on IMRT for head and neck cancer suggested significant heterogeneity in global head and neck IMRT practice patterns (17). For the same prescribed target dose and dose constraints for organ at risk, IMRT strategies in the study showed striking difference in various aspects, such as beam setup, total number of segments, PTV dose coverage and dose statistics for organs at risks. This European Society for Therapeutic Radiology and Oncology (ESTRO) planning exercise demonstrated that the planning of IMRT needs close cooperation between the various disciplines involved in the preparation and execution of a treatment.
It is believed that much of the heterogeneity in IMRT arises from physician-based factors. Clearly, radiation oncologists should receive sound training in those factors determined by the physician in performing IMRT, including target delineation, setting of parameters for objective functions, interpretation of the resultant plan, and understanding of uncertainties in delivery. It appears that there exists a lack of preparedness for fully implementing IMRT as a clinical routine in Korea. This study was therefore undertaken to assess the pattern of planning strategies for head and neck IMRT planning, with particular emphasis on physician-based factors such as identifying the target volume and non-tumor tissues on the planning CT, and specifying the dose to the target volume and the surrounding normal organs.
The factors such as RTPS, its optimization algorithm, beam data, and other physical QA-related issues were identically controlled, meaning that physician-related factors in IMRT planning (e.g., target segmentation and the prescription for inverse planning) have a greater influence on the resulting plan quality than QA issues in the delivery of complex IMRT plan. Although physical QA issues are also important, physician-based factors in IMRT planning are critical, and must undergo preparation before state-of-the art IMRT technology can be implemented in the clinical setting.
The present study identified marked variations in IMRT design and global patterns of planning strategies for IMRT of nasopharyngeal cancer in Korea. Substantial variations existed not only in target definition and dose prescription, but also with regard to the management of neck node areas; consequently, the resultant IMRT plans were strikingly different among the institutions. The variation in volume segmentation translated into the variation of dose statistics for standard ROIs. However, the dose statistics for individual institution's ROIs also showed substantial variation among institutions. This indicates that the difference of planning quality originates not only from the difference of ROI delineation but from the ability to perform inverse planning.
One of the shortcomings of the current study is that target volume determination guidelines and plan acceptance guidelines were not provided to each institution participated. It was remained to each institution's discretion. The IMRT could not be planned with only condition of doses of PTV1, PTV2 and PTV3 and without target determination guideline, acceptance criteria and so on. The marked variations in IMRT design and global patterns of planning results shown in the current study are not only due to the quality of IMRT planning in each institution but because of the lack of precise guideline while performing the IMRT planning. However, the institutions participated are in substantial agreement that the cause of wide variation of planning results are mainly due to variation of physician-based factors such as volume segmentation and setting the parameters for inverse planning. This wide variation could potentially be translated into different treatment outcomes between each institution.
Preparedness in performing IMRT is a crucial factor in its implementation. In the present study, the planning results of Institution 1 and 2 were relatively difficult to brag out. At the time of this planning study, Institution 1 and 2 did not performed IMRT for clinical cases. The attainment of excellence in IMRT practice requires greater effort in terms of time, man-power, and education, among other factors. Given the growing popularity of IMRT, it is not surprising that numerous IMRT "schools", seminars, and workshops have appeared internationally. However, there are few educational opportunities available for participating radiation oncologists in Korea. The human resources available to the KOSTRO radiation oncology society remain limited (10).
It is also necessary to support efforts to develop 'class-solution', in which an automated inverse planning protocol using a single set of inverse-planning parameters can be used for most patients to generate an acceptable IMRT plan. This would eliminate the need for time-consuming user-interface optimization in most cases. The adoption of computer-based assistance for physician factors in IMRT is also desirable (18).
Figures and Tables
Fig. 1
Variation in the delineation of GTV (A), PTV1 (B), PTV2 (C) among institutions.
Color key: red, institution 1; green, institution 2; blue, institution 3; yellow, institution 4; magenta, institution 5.
GTV, gross tumor volume; PTV, planning target volume.
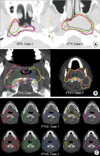
Fig. 2
Variation in the parotid gland delineation among institutions.
Color key: red, institution 1; green, institution 2; blue, institution 3; yellow, institution 4; magenta, institution 5.
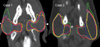
Fig. 5
Variation of the dose volume histogram for planning target volumes among participating institutions (Case 2).
Color key: red, institution 1; green, institution 2; blue, institution 3; yellow, institution 4; magenta, institution 5.

Table 6
Dose statistics for standard ROIs and each institution's ROIs (Case 1)

ROI, region of interest; PTV, planning target volume; D95, the dose where the cumulative DVH intersects with 95% of the volume; D50, the dose where the cumulative DVH intersects with 50% of the volume; D5, the dose where the cumulative DVH intersects with 5% of the volume; DVH, dose volume histogram.
References
1. Intensity Modulated Radiation Therapy Collaborative Working Group. Intensity-modulated radiotherapy: current status and issues of interest. Int J Radiat Oncol Biol Phys. 2001. 51:880–914.
2. Ezzell GA, Galvin JM, Low D, Palta JR, Rosen I, Sharpe MB, Xia P, Xiao Y, Xing L, Yu CX. Guidance document on delivery, treatment planning, and clinical implementation of IMRT: report of the IMRT Subcommittee of the AAPM Radiation Therapy Committee. Med Phys. 2003. 30:2089–2115.

3. Lee SW, Back GM, Yi BY, Choi EK, Ahn SD, Shin SS, Kim JH, Kim SY, Lee BJ, Nam SY, Choi SH, Kim SB, Park JH, Lee KK, Park SH, Kim JH. Preliminary results of a phase I/II study of simultaneous modulated accelerated radiotherapy for nondisseminated nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006. 65:152–160.

4. Lee SW, Kim GE, Suh CO, Chu SS, Lee KK, Moon SR. Intensity modulation technique using the complementary boost-fields for ethmoid sinus cancer. Clin Oncol (R Coll Radiol). 2002. 14:241–249.

5. Lee N, Xia P, Quivey JM, Sultanem K, Poon I, Akazawa C, Akazawa P, Weinberg V, Fu KK. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002. 53:12–22.

6. Chao KS, Ozyigit G, Tran BN, Cengiz M, Dempsey JF, Low DA. Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2003. 55:312–321.

7. Eisbruch A, Marsh LH, Dawson LA, Bradford CR, Teknos TN, Chepeha DB, Worden FP, Urba S, Lin A, Schipper MJ, Wolf GT. Recurrences near base of skull after IMRT for head-and-neck cancer: implications for target delineation in high neck and for parotid gland sparing. Int J Radiat Oncol Biol Phys. 2004. 59:28–42.

8. Mendenhall WM, Amdur RJ, Palta JR. Intensity-modulated radiotherapy in the standard management of head and neck cancer: promises and pitfalls. J Clin Oncol. 2006. 24:2618–2623.

9. Kam MK, Leung SF, Zee B, Chau RM, Suen JJ, Mo F, Lai M, Ho R, Cheung KY, Yu BK, Chiu SK, Choi PH, Teo PM, Kwan WH, Chan AT. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007. 25:4873–4879.

10. Huh SJ. Korean Society of Therapeutic Radiology and Oncology (KOSTRO). Current status of the infrastructure and characteristics of radiation oncology in Korea. Jpn J Clin Oncol. 2007. 37:623–627.

11. Kim JY, Kim DY, Kim TH, Park SY, Lee SB, Shin KH, Pyo H, Kim JY, Cho KH. Intensity-modulated radiotherapy with a belly board for rectal cancer. Int J Colorectal Dis. 2007. 22:373–379.

12. Choi Y, Kim JK, Lee HS, Hur WJ, Chai GY, Kang KM. Impact of intensity-modulated radiation therapy as a boost treatment on the lung-dose distributions for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2005. 63:683–689.

13. Choi Y, Kim JK, Lee HS, Hur WJ, Hong YS, Park S, Ahn K, Cho H. Influence of intravenous contrast agent on dose calculations of intensity modulated radiation therapy plans for head and neck cancer. Radiother Oncol. 2006. 81:158–162.

14. Okunieff P, Morgan D, Niemierko A, Suit HD. Radiation dose-response of human tumors. Int J Radiat Oncol Biol Phys. 1995. 32:1227–1237.

15. Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys. 1989. 16:1623–1630.
16. Burman C, Kutcher GJ, Emami B, Goitein M. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys. 1991. 21:123–135.

17. Claus F, Mijnheer B, Rasch C, Bortfeld T, Fraass B, De Gersem W, Wirtz H, Hoinkis C, Cho BC, Kwong LW, Bae H, Muller K, De Neve W. Report of a study on IMRT planning strategies for ethmoid sinus cancer. Strahlenther Onkol. 2002. 178:572–576.

18. Chao KS, Bhide S, Chen H, Asper J, Bush S, Franklin G, Kavadi V, Liengswangwong V, Gordon W, Raben A, Strasser J, Koprowski C, Frank S, Chronowski G, Ahamad A, Malyapa R, Zhang L, Dong L. Reduce in variation and improve efficiency of target volume delineation by a computer-assisted system using a deformable image registration approach. Int J Radiat Oncol Biol Phys. 2007. 68:1512–1521.





 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download