Abstract
We aimed to evaluate the feasibility of concurrent chemoradiotherapy (CRT) with capecitabine and cisplatin in patients with squamous cell carcinoma of the esophagus. Eighteen patients with esophageal cancer were enrolled on the study. The chemotherapy during CRT consisted of two cycles of intravenous cisplatin of 60 mg/m2 on day 1 and oral capecitabine 825 mg/m2 twice daily from day 1 to 14 at 3-week intervals. The radiotherapy (2.0 Gy fraction/day to a total dose of 60 Gy) was delivered to the primary tumor site and regional lymph node. After concurrent CRT, 2 cycles of capecitabine (1,000 mg/m2 b.i.d from days 1 to 14) plus cisplatin (60 mg/m2 on day 1) were added every 3 weeks. All patients completed the planned treatment. After the chemoradiotherapy, 12 complete responses (CR, 66.7%) and 6 partial responses (PR, 33.3%) were confirmed. Grade 3 or 4 neutropenia only occurred in 2 patients, plus no treatment-related death was observed. At a median follow-up duration of 14.9 months, the estimated overall survival and progression-free survival rate at 2-yr was 70.7% and 54.4%, respectively. Concurrent CRT with capecitabine and cisplatin was found to be well-tolerated and effective in patients with esophageal cancer.
During the past 20 yr, preoperative or definitive chemoradiotherapy (CRT) for esophageal carcinoma has revealed promising results (1-4). After the report of an intergroup randomized controlled trial (Radiation Therapy Oncology Group 85-01), which compared CRT with radiotherapy alone, the combined modality treatment became a standard for patients who received nonsurgical treatment for esophageal cancer (3, 4).
Generally, a combination of 5-fluorouracil (5-FU) and cisplatin has been considered as effective regimen for concurrent CRT due to the synergism between the two agents and their radiosensitizing effects as well as its clinical outcome (5, 6). However, the adverse effects of 5-FU, such as esophagitis, which is an additive complication to radiation, or bone marrow suppression, can result in treatment-related hospitalization or mortality, thereby compromising the quality of life and compliance to treatment (3, 4).
The oral fluoropyrimidine capecitabine (Xeloda®; Hoffmann-La Roche) was rationally designed to preferentially generate 5-FU in tumor tissue and mimic continuous-infusion 5-FU. This tumor selectivity is achieved through exploiting the significantly higher activity of thymidine phosphorylase (TP) in many tumor tissues compared with healthy tissue (7, 8). The expression of this enzyme is enhanced in tumor areas with poor perfusion, hypoxia, and acidosis, a situation found in most advanced esophageal. Moreover, there is evidence that radiation leads to the up regulation of TP expression (9). In a preclinical study, capecitabine given orally resulted in consistently higher tissue-to-plasma 5-FU concentration ratios than 5-FU administered intravenously (10). In addition, capecitabine has also exhibited antitumor activity when given as a monotherapy or in combination with other agents in patients with various solid tumors as well as advanced esophageal cancer (11-14).
Furthermore, since the key side effects of capecitabine are hand-foot syndrome and diarrhea, which overlap little with the side effects of cisplatin or radiation, capecitabine can be a good chemotherapeutic agent in concurrent CRT for esophageal cancer.
Accordingly, the current study was conducted to evaluate the feasibility of definitive CRT with capecitabine and cisplatin for esophageal cancer.
All the patients involved in the current study had histologically confirmed esophageal squamous cell carcinoma. The patients were 20-75 yr of age with a performance status of 0-2 on the Eastern Cooperative Oncology Group (ECOG) scale. Plus, adequate hematological (WBC count ≥4×109/L, platelet count ≥100×109/L, hemoglobin ≥9 g/dL), renal (serum creatinine ≤1.5 mg/dL and creatinine clearance ≥50 mL/min), and hepatic (total bilirubin ≤2.0 mg/dL and serum transaminase level ≤3 times the upper limit of the normal range) levels were also required. Patients were ineligible if they had previously received chemotherapy or radiation therapy, or had other severe medical illnesses, distant metastasis, another active malignancy in the last 5 yr, except treated nonmelanoma skin cancer or cervical dysplasia, or a history of anaphylaxis to drugs. The institutional review board of the authors' institution approved the protocol, and written informed consent was obtained from all patients before enrollment.
The administration schedule is shown in Fig. 1. The CRT consisted as follows: Capecitabine 825 mg/m2 b.i.d was given on days 1 to 14. The capecitabine was supplied as film-coated tablets at two dose strengths, 150 mg and 500 mg, while the cisplatin 60 mg/m2 was administered through a 1-hr intravenous infusion on the first day of each cycle. Pre and post intravenous hydration and appropriate antiemetics were also administered to prevent renal toxicity and emesis. Two cycles of chemotherapy were repeated every 3 weeks. Radiotherapy (2.0 Gy fraction/day to a total dose of 60 Gy), administered 5 days per week, was delivered to the primary tumor site and regional lymph node, and was targeted to begin on the first day of chemotherapy. Every effort was made to continue the radiation on schedule.
After concurrent CRT, 2 cycles of capecitabine (1,000 mg/m2 b.i.d from days 1 to 14, followed by a 7-day rest) plus cisplatin (60 mg/m2 on day 1) were added every 3 weeks.
The protocol plan was to continue the study treatment despite esophagitis or dermatitis. However, if grade 3 or 4 capecitabine-related hematological or non-hematological toxicity, such as diarrhea and hand-foot syndrome, occurred (not including radiation-related toxicity), the capecitabine was with-held until the toxicity had improved by at least two grade levels. Subsequent capecitabine doses then required a 20% dose reduction. The dose of cisplatin was reduced to 50% if the calculated creatinine clearance level was 30 to 50 mL/min. No cisplatin was administered if the creatinine clearance level was less than 30 mL/min. In the presence of myelosuppression (WBC count <4×109/L or platelet count <100×109/L), a persisting fever that exceeded 38℃, or other clinically apparent infections, a cycle could be postponed for 1 week or interrupted if this was judged to be necessary in the opinion of the attending physicians.
Before being enrolled on the trial, all patients underwent a full medical history and physical examination, blood tests, esophagoscopy, and computed tomography (CT). Assessment of the tumor response by CT scanning and an endoscopic biopsy to determine the pathologic response were performed 4 weeks after completing the study treatment. The definitions of complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were based on the response evaluation criteria in solid tumors (RECIST) guidelines (15). The patients were monitored for toxicity (medical interview, physical examination, and complete blood count) throughout the treatment. Complete blood counts and chemistry were performed every week until the end of the chemoradiotherapy. Systemic toxicity resulting from treatment was graded according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3.0. Acute radiation toxicities were graded according to the European Organization Therapy Oncology Group (EORTC-RTOG) toxicity criteria. Hand-foot syndrome was graded 1 to 3, as defined in previous capecitabine clinical studies (12).
For sample size calculation, the current trial used a two-stage optimal design with a 90% power to accept the hypothesis and 5% significance to reject the hypothesis. Plus, the current trial was designed to detect a response rate of 90% as compared to a minimal, clinically meaningful response rate of 70%. Allowing for a follow-up loss rate of 10%, the total sample size was 36 patients with a measurable disease. After enrollment of 18 patients, we planned to report the feasibility results as a pilot study. Overall survival was measured from the initiation of therapy to the date of the last follow-up or any cause of death. Progression-free survival was measured as the time from the initiation of therapy until death of disease or toxicity, appearance of new lesions, or a greater than 25% increase of the indicator lesions over the previous smallest size. Progression-free and overall survival analyses were all estimated using the Kaplan-Meier method. The statistical data were obtained using an SPSS software package (SPSS 11.0 Inc. Chicago, IL, U.S.A.).
A total of 18 patients were enrolled in the current study from July 2004 to January 2006 at Kyungpook National University Hospital, Daegu, Korea. The characteristics of the patients are summarized in Table 1. The median age of the patients was 68.0 yr (range, 59-75 yr), and 17 (94.4%) patients were male. All the patients had a good performance status (ECOG 1). The lower esophagus (77.8%) was the most common primary tumor site. All patients had squamous cell carcinoma on histology. Twelve patients (66.7%) had a stage II disease, while the remaining 6 patients were stage III. Ten patients (44.5%) had an N1 status before treatment.
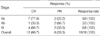
All patients completed the concurrent CRT and 2 cycles of chemotherapy. After the chemoradiotherapy, 12 complete responses (CR, 66.7%) and 6 partial responses (PR, 33.3%) were confirmed (Table 2). Among the 6 patients who failed to achieve CR after the chemoradiotherapy, 5 patients received salvage chemotherapy with taxanes. At the time of the present evaluation, 7 patients (6 patients with PR, 1 patient with CR) had developed disease progression or recurrence (2-primary tumor, 1-regional lymph node, 1-both primary tumor and regional lymph node, and 3-distant metastases to the bone, liver, brain), and 4 patients had died of disease progression. The median survival time had not yet been reached at a median follow-up duration of 14.9 months (range, 5.9-27.9 months), while the estimated overall survival and progression-free survival rate at 2-yr was 70.7±13.0% and 54.4±13.2%, respectively (Fig. 2). Locoregional control rate of the disease at 2-yr was 69.6±13.6%.
All patients were assessable for toxicity. The hematologic and non-hematologic toxicities that occurred during the current study are summarized in Table 3. The most severe hematologic adverse event was neutropenia, which occurred with a grade 3 intensity in 2 patients (11.1%). However, no febrile neutropenia and no treatment-related death occurred during this study. Esophagitis and dermatitis, as expected from a combination of radiation with an effective chemotherapeutic-sensitizing agent, were the common non-hematological toxicities. Grade 3/4 esophagitis and dermatitis was observed in 27.8% and 16.7%, respectively. Four patients with severe esophagitis needed parenteral nutrition support, and the concurrent radiotherapy was interrupted in 2 patients (for 7 days and 5 days, respectively). Grade 2 hand-foot syndrome, a complication of capecitabine, only occurred in 2 patients (11.1%). The dose of capecitabine was reduced in 2 cycles due to neutropenia or diarrhea, and cisplatin omitted from 1 cycle because of nephrotoxicity. The second cycle of chemotherapy was delayed in 5 patients for the following reasons: hematological toxicity (n=4) and patient refusal (n=1). During the 2 cycles of chemotherapy after the concurrent CRT, the dose of capecitabine was reduced in 6 cycles due to neutropenia, diarrhea, or fatigue, and the chemotherapy was delayed in 6 patients (hematological toxicity [n=5], non-hematological toxicity [n=1]). The dose intensity of capecitabine/cisplatin was 96.2%/99.4% in cycle 1 and 92.1%/97.2% in cycle 2, respectively.
The present study was designed to evaluate the feasibility of capecitabine instead of 5-FU, a commonly used agent, in combination with cisplatin for concurrent chemoradiotherapy in patients with esophageal cancer. In the current study, the clinical CR rate (66.7%), locoregional control rate (69.6% at 2-yr), and progression-free survival rate (54.4% at 2-yr) following treatment with the present regimen, which can be administered on an outpatient basis, were comparable with previous results reported for 5-FU and platinum-based concurrent chemoradiotherapy, although the follow-up period was relatively short to compare the survival rate directly (3, 4, 16-18). For example, concurrent chemotherapy with infusion of 5-FU and cisplatin arm achieved a 2-yr and 5-yr overall survival rate of 38% and 27%, respectively, in a randomized study compared with concurrent chemoradiotherapy with radiation therapy alone (3, 4). Ishikura et al. (18) also reported that the definitive CRT with 5-FU and cisplatin exhibited a CR rate of 56% and 5-yr overall survival rate of 29% in 139 patients with thoracic esophageal cancer.
Since the efficacy and favorable safety profile of capecitabine have been clearly demonstrated in recent large phase III studies comparing capecitabine with intravenous 5-fluorouracil plus leucovorin for metastatic colorectal cancer (13, 19), capecitabine has been widely used in the treatment of breast cancer, stomach cancer, and other solid tumors (11, 12, 20). Capecitabine also offers a number of potential advantages as a chemoradiosensitizer in concurrent chemoradiotherapy. Daily administration mimicking the continuos infusion of 5-FU can act as a radiosensitizer for every fraction of radiotherapy. Furthermore, its mode of activation by TP and radiotherapy concentrates it within tumor cells, raising the prospect of better tumor control. Given these advantages, several studies have demonstrated that concurrent chemoradiotherapy using capecitabine, with a dose ranging from 800 mg/m2 to 825 mg/m2 b.i.d, in combination with cisplatin or oxaliplatin is effective and has a low toxicity profile in the treatment of rectal cancer or locally advanced head and neck cancer (21-23).
Esophagitis and myelosuppression are the most serious complications of concurrent chemoradiotherapy for esophageal cancer, resulting in a reduced compliance to treatment or sometimes mortality. In the present study, esophagitis was the common adverse effect observed, with a grade 3 or 4 intensity in 27.8% of the patients. In a randomized study by Herskovic et al. (3, 4), the incidence of grade 3 or 4 esophagitis in concurrent chemoradiotherapy with 5-FU and cisplatin was 20.0%, which was higher than with radiotherapy only (4.8%). The incidence of esophagitis was not so different between chemoradiotherapy with capecitabine/cisplatin and 5-FU/cisplatin. Meanwhile, grade 3 or 4 leukopenia only occurred in 2 patients (11.1%), plus no febrile neutropenia was observed in the current study. These incidences of hematologic toxicities were lower than previous studies using 5-FU-containing regimens, where the incidence of grade 3 or 4 leukopenia was 33.3% to 43.0% (16-18).
In conclusion, concurrent chemoradiotherapy with capecitabine and cisplatin was found to be well-tolerated and seemed to be effective in patients with esophageal cancer. However, a multicenter phase II or phase III study is needed to evaluate the role of capecitabine compared to 5-FU in the CRT for esophageal cancer.
Figures and Tables
References
1. Coia LR. Chemoradiation as primary management of esophageal cancer. Semin Oncol. 1994. 21:483–492.
2. Forastiere AA, Orringer MB, Perez-Tamayo C, Urba SG, Zahurak M. Preoperative chemoradiation followed by transhiatal esophagectomy for carcinoma of the esophagus: final report. J Clin Oncol. 1993. 11:1118–1123.

3. Herskovic A, Martz K, Al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, Cooper J, Byhardt R, Davis L, Emami B. Combined chemotherapy and radiotherapy in patients with cancer of the esophagus. N Engl J Med. 1992. 326:1593–1598.
4. Al-Sarraf M, Martz K, Herskovic A, Leichman L, Brindle J, Vaitkevicius V, Cooper J, Byhardt R, Davis L, Emami B. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer. An intergroup study. J Clin Oncol. 1997. 15:277–284.

5. Scanlon KJ, Newman EM, Lu Y, Priest DG. Biochemical basis for cisplatin and 5-fluorouracil synergism in human ovarian carcinoma cells. Proc Natl Acad Sci USA. 1986. 83:8923–8925.

6. Byfield JE. Lokich JJ, editor. Combined modality infusional chemotherapy with radiation. Cancer Chemotherapy by Infusion. 1990. 2nd ed. Chicago, IL: Percepta Press;521–551.
7. Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998. 34:1274–1281.

8. Schuller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, Utoh M, Mori K, Weidekamm E, Reigner B. Preferential activation of capecitabine in tumor following oral administration in colorectal cancer patients. Cancer Chemother Pharmacol. 2000. 45:291–297.
9. Sawada N, Ishikawa T, Sekiguchi F, Tanaka Y, Ishitsuka H. X-Ray irradiation induces thymidine phosphorylase and enhances the efficacy of capecitabine (Xeloda) in human xenografts. Clin Cancer Res. 1999. 5:2948–2953.
10. Ishikawa T, Utoh M, Sawada N, Nishida M, Fukase Y, Sekiguchi F, Ishitsuka H. Tumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts. Biochem Pharmacol. 1998. 55:1091–1097.

11. Kim TW, Kang YK, Ahn JH, Chang HM, Yook JH, Oh ST, Kim BS, Lee JS. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced gastric cancer. Ann Oncol. 2002. 13:1893–1898.

12. Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, Osterwalder B, Burger HU, Brown CS, Griffin T. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999. 17:485–493.

13. Van Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta F, Boyer M, Bugat R, Findlay M, Frings S, Jahn M, McKendrick J, Osterwalder B, Perez-Manga G, Rosso R, Rougier P, Schmiegel WH, Seitz JF, Thompson P, Vieitez JM, Weitzel C, Harper P. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001. 19:4097–4106.
14. Lorenzen S, Duyster J, Lersch C, von Delius S, Hennig M, Bredenkamp R, Peschel C, Lordick F. Capecitabine plus docetaxel every 3 weeks in first- and second-line metastatic oesophageal cancer: final results of a phase II trial. Br J Cancer. 2005. 92:2129–2133.

15. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, Van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000. 92:205–216.
16. Ohtsu A, Boku N, Muro K, Chin K, Muto M, Yoshida S, Satake M, Ishikura S, Ogino T, Miyata Y, Seki S, Kaneko K, Nakamura A. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol. 1999. 17:2915–2921.

17. Ishikura S, Nihei K, Ohtsu A, Boku N, Hironaka S, Mera K, Muto M, Ogino T, Yoshida S. Long-term toxicities after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2002. 21:2697–2702.
18. Ishida K, Ando N, Yamamoto S, Ide H, Shinoda M. Phase II study of cisplatin and 5-fluorouracil with concurrent radiotherapy in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG)/Japan Clinical Oncology Group Trial (JCOG9516). Jpn J Clin Oncol. 2004. 34:615–619.

19. Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, Maroun J, Walde D, Weaver C, Harrison E, Burger HU, Osterwalder B, Wong AO, Wong R. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001. 19:2282–2292.

20. Kim TW, Chang HM, Kang HJ, Lee JR, Ryu MH, Ahn JH, Kim JH, Lee JS, Kang YK. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced biliary cancer. Ann Oncol. 2003. 14:1115–1120.

21. Rodel C, Grabenbauer GG, Papadopoulos T, Hohenberger W, Schmoll HJ, Sauer R. Phase I/II trial of capecitabine, oxaliplatin, and radiation for rectal cancer. J Clin Oncol. 2003. 21:3098–3104.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download