Abstract
This study was performed in order to characterize the types of the infiltrating cells, and the expression profiles of major histocompatibility complex (MHC) class I and membrane attack complex (MAC) in patients with inflammatory myopathies and dysferlinopathy. Immunohistochemical stains were performed using monoclonal antibodies against several inflammatory cell types, MHC class I, and MAC in muscles from inflammatory myopathies and dysferlinopathy. There was significant difference in the types of infiltrating cells between polymyositis (PM), dermatomyositis (DM), and dysferlinopathy, including significantly high CD4+/CD8+ T cell ratio and B/T cell ratio in DM. In dysferlinopathy, CD4+ T cells were the most abundant and the proportions of infiltrating cell types were similar to those of DM. MHC class I was expressed in muscle fibers of PM and DM regardless of the presence of inflammatory infiltrates. MAC was expressed in necrotic fibers and vessels of PM and DM. One patient with early stage DM had a MAC deposits on endomysial capillaries. In dysferlinopathy, MAC deposit was also observed on the sarcolemma of nonnecrotic fibers. The analysis of inflammatory cells, MHC class I expressions and MAC deposits may help to differentiate dysferlinopathy from idiopathic inflammatory myopathy.
Polymyositis (PM) and dermatomyositis (DM) are the two most common idiopathic inflammatory myopathies. They have in common the presence of moderate to severe muscle weakness and inflammation in the muscle, and the diagnosis is based on combination of clinical examination, electromyographic data, serum muscle enzyme levels, and muscle biopsy findings (1). In muscle biopsy, they are histopathologically characterized by the presence of mononuclear cellular infiltrates in skeletal muscle tissue. Previous studies also have revealed a significant difference in the pathogenesis of inflammatory myopathies, such as a complement-mediated injury directed against the intramuscular microvasculature in DM or T-cell-mediated cytotoxicity against major histocompatibility complex (MHC) class I expressing muscle fibers in PM, showing distinctive differences in the types and patterns of infiltrating mononuclear cells (2, 3). In some subsets of patients with PM or DM, however, muscle biopsy may fail to show any significant inflammatory reactions, and may even appear completely normal.
On the other hand, inflammatory cell infiltration could be a prominent feature in dysferlinopathy, a group of muscular dystrophies inherited as an autosomal recessive trait (4). Dysferlinopathy is caused by a mutation in DYSF and produces the deficiency of dysferlin in the sarcolemma as demonstrated by immunocytochemistry (5, 6). There are two important allelic diseases in dysferlinopathy-one with predominant proximal limb muscle weakness (limb-girdle muscular dystrophy type 2B [LGMD2B], MIM, #253601), and the other with prominent distal leg muscle weakness (Miyoshi myopathy [MM], MIM #254130) (4). Despite the distinct patterns of muscle involvement, they have many features in common such as very high serum CK level and onset in late teens. When muscle biopsy from patients with dysferlinopathy shows prominent inflammatory changes, an incorrect diagnosis of an inflammatory myopathy can be made, and can leads to inappropriate treatment and care in clinical practice.
Recently, the immunohistochemical stains have been used to make up the limitation of conventional light microscopy in the differential diagnosis of inflammatory myopathies and muscular dystrophies with inflammatory changes. It also enables us to analyze infiltrating mononuclear cells and identify immunological markers, such as MHC class I or membrane attack complex (MAC).
The present study has been designed to characterize the types and patterns of the infiltrating mononuclear cells and the expression profiles of MHC class I and MAC in patients with idiopathic inflammatory myopathies (PM and DM) and dysferlinopathy with inflammatory cell infiltration.
Between 2004 and 2006, we had muscle biopsies from 32 patients with idiopathic inflammatory myopathies and four patients with dysferlinopathy (one patient with LGMD2B and three with MM). Among patients with inflammatory myopathies, 13 patients (nine patients with PM and four with DM) were finally selected for the analysis according to the following inclusion criteria: 1) A detailed clinical record should be available, 2) clinical and laboratory findings should fulfill the Bohan and Peter's diagnostic criteria of PM and DM (1), 3) immune modulating therapy showed definite clinical improvement, 4) enough amount of frozen muscle specimen is available for the immunohistochemical study, and 5) normal dysferlin expression in muscle biopsy.
The diagnosis of dysferlinopathy was confirmed by the selective loss of dysferlin in immunohistochemical stain using anti-dysferlin antibodies (Clone Ham1/7B6, Novocastra Laboratories Ltd, Newcastle upon Tyne, U.K.). The detailed methods of dysferlin immunohistochemistry had been described previously (7).
Then, we have divided our patients into four groups on the basis of clinical and pathological features. Patient's clinical data are summarized in Table 1. Also, five normal muscle specimens were obtained from patients undergoing orthopedic operations with written informed consents, and served as controls for immunohistochemical studies.
The first group consisted of five patients fulfilling both clinical and histopathological features according to Bohan and Peter's criteria. All had a symmetrical weakness of proximal limbs, except for one who initially presented with an initial muscle weakness of bilateral forearms and hands, which eventually progressed to limb-girdle and neck flexor muscles (patient 1). Symptom duration varied between 3 to 12 months except for one who had a five year history of progressive proximal muscle weakness (patient 3). Histopathologically, all patients showed clear inflammatory cell infiltrates as well as necrosis and regeneration of muscle fibers in their muscle tissues. They all had an elevated CK level and active myopathic patterns on needle electromyography. Immunosuppressive therapy was performed in all, either with prednisolone only or in combination with azathioprine. All of them showed clinical improvement after treatment.
The second group consisted of four patients with definite clinical features of myositis, but without detectable infiltration of inflammatory cells in their muscle biopsies. They all presented with a progressive and symmetrical weakness of proximal limbs, and showed an elevated CK level and active myopathic features on electromyography. Despite clinical symptoms and laboratory findings of myositis, the histopathological evaluation of the muscle biopsies did not reveal inflammatory infiltrates typical of myositis, and there were only nonspecific myopathic findings, such as atrophy, necrosis or regeneration of muscle fibers in their muscle biopsies. Symptom duration varied between 2 to 4 months with a median duration of 3 months. One patient was found to have an advanced gastric cancer at the time of the diagnosis (patient 7). Two patients received immunosuppressive therapy with prednisolone and intravenous immunoglobulin therapy (patient 6, 7). The others were treated with prednisolone alone (patients 8, 9). All the patients in this group also responded to the treatment.
This group consisted of four patients with dermatomyositis according to Bohan and Peter's criteria. Three patients fulfilled the criteria for definite myositis. One patient (patient 12) presented with myalgia rather than weakness in limbs, and serum CK level was normal. However, she had a lilac discolorization of the eyelids (heliotrope) with periorbital edema and facial rash, and fulfilled the criteria of electromyographic study and histopathological findings for myositis, and was diagnosed as probable dermatomyositis based on Bohan and Peter criteria. Histopathologically, all patients had characteristic findings of dermatomyositis, such as perifascicular atrophy and inflammatory infiltrations in perimysium or perivascular region. One patient (patient 10) was a 6-yr-old girl with a shorter duration of symptoms than the others. All patients received immunosuppressive therapy with prednisolone alone, and showed a clinical improvement.
This group consisted of three patients with MM and one with LGMD2B. All of them were previously diagnosed as dysferlinopathy on the basis of a selective deficiency of dysferlin in their muscle biopsies. Three patients (patients 14, 15, 16) were compatible with a phenotype of MM with high CK levels and predominant distal lower leg weakness which had been progressive worse over years. The patient 15 had been erroneously diagnosed and treated as PM on the basis of routine histochemistry findings of muscle biopsy at another hospital. Another patient (patient 17) was a 21-yr-old female with progressive proximal limb muscle weakness for two years with high serum CK level. Among four patients with dysferlinopathy, two (patient 15 and 16) showed an inflammatory change in their muscle biopsies.
All muscle specimens were obtained by diagnostic muscle biopsy which had been performed before the treatment, and were frozen and stored at -70℃ until used. For immunohistochemical analyses, serial 7 µm cryostat sections were cut and mounted on gelatin-coated glass slides at -20℃, and air-dried for 30 min at room temperature. The first and the last sections were stained with hematoxylin and eosin in order to confirm that the configuration of the sections were unchanged throughout the serial sections collected for immunohistochemistry. Sections were fixed in acetone for 5 min and then rinsed in phosphate buffered saline (PBS). Endogenous peroxidase was blocked with 3% H2O2 for 10 min. After rinsed by PBS for 10 min, sections were incubated for 60 min with primary antibodies. Mouse monoclonal antibodies were all purchased from Dako (Glostrup, Denmark) (Table 2), and were used to identify CD8+ T cells, CD4+ T cells, B cells (CD20), macrophages (CD68), MHC class I (HLA-ABC) and C5b-9 complement deposits (membrane attack complex, MAC). After washing the specimens with PBS for 5 min three times, the secondary antibodies coupled with peroxidase molecules were applied for 30 min. Subsequently, it was incubated with a chromogenic substrate solution for peroxidase 3,3'-diaminobenzidine tetrahydrochloride (DAB) (Rabbit/Mouse Ig Immunohistochemistry Kit, EnVision™+Detection System, DAKO, Glostrup, Denmark). After a final rinse, the sections were counterstained with hematoxylin and mounted.
For quantitation of inflammatory infiltrates, CD8+ and CD4+ T cells, B cells and macrophages were counted in serial sections under the light microscope. Two different clusters were chosen for each patient and the number of cells in each cell type was manually counted. The clusters were randomly selected from the groups of cells surrounding or invading muscle fibers under×100 magnification, and the results of counting were compared with those in each group (Group 1, 3, 4). MHC class I and MAC were assessed as to the distribution and intensity of the staining.
As shown in Table 3, in the patients with PM (group 1), abundant CD8+ T cells and macrophages were observed, accounting for 36.6±12.7% and 32.0±15.2% of the total cells counted, respectively. They were mainly observed as clusters or scattered in endomysial spaces, but were also found in perimysial and perivascular sites. CD4+ T cells were less abdundant, accounting for 25.6±6.6% and were detected mainly in perimysial or perivascular sites (Fig. 1A-E). The most interesting finding was that a number of non-necrotic fibers were focally surrounded and invaded by predominantly CD8+ T cells (Fig. 1B). On the other hand, necrotic fibers were invaded by CD4+ T cells and macrophages as well as CD8+ T cells. Only a few B cells were detectable mainly in perivascular sites.
In the patients with DM (group 3), inflammatory cells were mainly found in perimysial and perivascular sites, and CD4+ T cells and macrophages were more frequent, accounting for 36.6±9.4% and 36.9±9.1% of the total, respectively. B cells were more abundant than in PM patients (Fig. 1F-J). In addition, there were significant differences in CD4+/CD8+ T cell ratio and B/T cell ratio between PM and DM (0.8±0.4% vs. 4.8±4.1%; P=0.001, and 0.10±0.06% vs. 0.32±0.23%; P=0.009).
In the patients with dysferlinopathy (group 4, patient 15 and 16), the types and patterns of the inflammatory cells were similar to those of DM patients (group 2), such as the high CD4+/CD8+ T cell ratio and the high percentage of B cells. CD4+ T cells were most abundant, accounting for 42.2±7.7% of the total, and were mainly observed in perivascular sites (Fig. 1K-O).
The MHC class I reactivity was clearly expressed on normal-looking fibers as well as degenerating fibers in PM (Fig. 2B-D) and DM (Fig. 2G, H) patients with prominent inflammatory cellular infiltrates. The non-necrotic fibers showed a marked sarcolemmal reactivity for MHC class I. In some patients, pronounced cytoplasmic staining was present in nonnecrotic fibers as well as necrotic ones. In addition, MHC class I was strongly expressed in the extracellular matrix, endothelial cells of capillaries, arterioles and venules. There was no significant difference in MHC class I expression between PM and DM patients. However, the perifascicular areas tended to be strongly stained with cytoplasmic reactivity in DM patients. Interestingly, the expression of MHC class I was also up-regulated in normal-looking fibers in the patients with PM without inflammatory infiltrates (Fig. 2E, F). In this group of the patients, a varying pattern and intensity of the staining was observed; MHC class I expression was restricted to the sarcolemma in three patients whereas it was also observed within their cytoplasm in one patient (patient 6). In the patients with dysferlinopathy, there was no or only mild expression of MHC class I on the sarcolemma (Fig. 2I, L).
MAC deposits were found within necrotic fibers and vessel walls in both PM and DM patients (Fig. 3A, B). Especially, in a 6-yr-old girl with DM who had a disease duration of three months (patient 10), there were MAC deposits on endomysial capillaries as well (Fig. 4A, B). In other patients with DM, by contrast, no endomysial MAC deposit was observed (Fig. 4C, D). In the patients with dysferlinopathy, MAC deposits were detected in necrotic fibers and vessel walls, too. Interestingly, three of the four dysferlinopathy patients (patient 14, 16, 17) had sarcolemmal MAC deposits on nonnecrotic fibers, especially in the perimysial or perivascular spaces (Fig. 3C, D). However, sarcolemmal MAC deposits on nonnecrotic fibers were not present in patients with inflammatory myopathy.
The idiopathic inflammatory myopathies are autoimmune-mediated disorders, which is supported by their association with other autoimmune disorders, autoantibodies, and the evidence of T cell-mediated myocyotoxicity or complement-mediated microangiopathy (2, 3, 8). They are histologically characterized by the mononuclear cellular infiltrates in skeletal muscle tissue. However, the types and patterns of cellular infiltrates are distinct, probably due to the significant differences in autoimmune pathogenesis. This study again demonstrated differences in cell types and distributions of cellular infiltrates, and patterns of inflammation between PM and DM as previously studied (9-12). In PM, the inflammatory cells were found mainly in endomysial sites, and CD8+ T cells (cytotoxic) were most abundant and focally surrounded and invaded non-necrotic fibers expressing MHC class I antigen. These findings suggest that cytotoxic T-cell-mediated muscle fiber injuries occur through immune-mediated process. In DM, the inflammatory cells were predominantly found in perivascular and perimysial sites, and to a lesser extent, in endomysial site. In addition, they were mainly consisted of CD4+ T cells and B cells, suggesting a humorally mediated process.
Interestingly, inflammatory changes are frequent in muscle of patients with dysferlinopathies (4, 13), and similar inflammatory features have been found in SJL/J mice with DYSF mutation which also results in a lack of sarcolemmal dysferlin (14). Thus, when dysferlinopathy is associated with inflammatory infiltrates, it can not be easily distinguished from inflammatory myopathy by routine histochemistry. Under immunohistochemical studies, however, the types and patterns of inflammation seem to differ from that observed in inflammatory myopathies. In our study, inflammatory cells were mainly observed in perivascular sites, and CD4+ T cells were most abundant in patients with dysferlinopathy. Furthermore, CD8+ T cells were scarce and there was no non-necrotic fibers surrounded or invaded by CD8+ T cells. These findings indicate the cytotoxic T-cell-mediated immune mechanism directed against muscle fibers is unlikely in dysferlinopathy. In our study, dysferlinopathy shared several features with DM in terms of the types of inflammatory cellular infiltrates, including high CD4+/CD8+ T cell ratio and the high percentage of B cells. However, the expression of MHC class I and MAC deposits were significantly different in DM and dysferlinopathy, suggesting the inflammatory reactions in dysferlinopathy are not related with a specific immune mechanism as is seen in DM, although the origin and role of these inflammatory reactions remain unclear in dysferlinopathy.
In normal muscle fibers, MHC class I is not expressed on the sarcolemma. The abnormal expression of MHC class I always occurs in muscle biopsies from idiopathic inflammatory myopathies. An increased expression of MHC class I was observed in patients with chronic, inactive inflammatory myopathies as well as with active inflammatory myopathies (15-18). In addition, MHC class I is detectable even after the immunosuppressive treatment has been initiated (19). These findings suggest that the expression of MHC class I plays an important role in the pathogenesis of idiopathic inflammatory myopathies. In our study, a diffuse expression of MHC class I was observed in patients with idiopathic inflammatory myopathies as previously reported. It is interesting that the expression of MHC class I was not only observed in PM patients with active inflammatory infiltrates, but also in those without definite inflammatory infiltrates in their muscle biopsies. Their mean duration of disease after onset of symptom was 3 months, which is clearly shorter than that of our PM patients with prominent inflammatory infiltrates. Therefore, the expression of MHC class I might play a central role in mediating muscle weakness at the early stage of inflammatory myopathies, although further study will be needed in order to verify this assumption.
While it is widely accepted that MHC class I expression is up-regulated in inflammatory myopathies, it is not clear whether it is a result of the nonspecific response to muscle degeneration/regeneration or of the specific disease process itself. Previous reports have shown that MHC class I is induced by proinflammatory cytokines such as IFN-γ and TNF-α(20, 21), which are highly expressed in inflammatory myopathies. IL-1α also induces the expression of MHC class I on myotubes in vitro (22). Interestingly, the distribution of capillaries positive for IL-1α, and muscle fibers positive for MHC class I is identical in both chronic PM and DM patients (17). Recently, however, it had been hypothesized that the up-regulation of MHC class I is an initial event because a transgenic mice with conditionally up-regulated MHC class I has been led to self-sustained autoimmune myositis (22). It is supported by several studies that muscle fibers in inflammatory myopathies express MHC class I as well as class II independently of the inflammatory infiltrates, as was also observed in our study (23, 24).
In two patients with dysferlinopathy, there was no or only mild expression of MHC class I on the sarcolemma in spite of the inflammatory infiltrates. There had been one previous study which demonstrated the expression of MHC class I on the sacolemma of non-necrotic muscle fibers in dysferlinopathy with inflammation (25). However, the up-regulation of MHC class I was not accompanied by T-cell cytotoxicity, which is similar to the result of our study. In addition, knocking out the MHC class I gene in SJL/J mice does not alter the extent of myopathy, showing that it is not required for the development of myopathy (26). These findings indicate that MHC expression is not an essential feature in the pathogenesis of dysferlinopathy.
In dermatomyositis, the primary antigenic target is the endothelium of the endomysial capillaries. After the activation of complement, C5b-9 MAC is deposited on capillaries, which sequentially induce capillary necrosis, perivascular inflammation, ischemia, and destruction of muscle fibers (2, 8). However, no significant difference in MAC deposits between PM and DM was observed in this study. In addition, no MAC deposit on endomysial capillaries was seen in DM patients except for a girl with a shorter duration of the disease (patient 10). This might be explained by that the complement-mediated microvasculopathy is a primary pathogenic event only at the early stage of disease (27, 28). A recent study also showed that MAC deposits on capillaries were observed from patients who were within 1 to 3 months from the onset of childhood DM (29). These findings suggest that MAC deposits on endomysial capillaries could be a useful indicator for the diagnosis of DM at an early stage.
In three patients with dysferlinopathy, MAC deposits were also detected on sarcolemma of nonnecrotic fibers. This finding is not likely to be associated with inflammatory reactions because only one of them had inflammatory infiltrates and none of the patients with inflammatory myopathy had sarcolemmal MAC deposits. Although sarcolemmal MAC deposits on nonnecrotic fibers have been observed in some types of muscular dystrophy, the pathogenetic significance remains unclear. A recent study also failed to demonstrate the presence of sarcolemmal IgG or IgM that would be required to initiate the classic complement pathway, and C3 deposits that would be required to propagate the alternative pathway (30). However, the presence of sarcolemmal MAC deposits on nonnecrotic fibers may help to distinguish between a dysferlinopathy and an idiopathic inflammatory myopathy histologially.
Recently, it has been recognized that the clinical criteria of Bohan and Peter cannot exactly distinguish PM from inclusion body myositis (IBM) or certain muscular dystrophies (2, 3, 8). Thus, some patients with IBM or muscular dystrophies with inflammatory changes can be misdiagnosed and treated as PM, being exposed to the long-term side-effects of steroid and immunosuppressants. On the other hand, some patients with PM who fulfill the clinical criteria but without inflammatory reactions in muscle biopsy could be faced to a diagnostic dilemma. With the recent advances in the immunopathogenesis of inflammatory myopathies demonstrated by various immunohistochemical studies, the new diagnostic criteria were recently introduced, in which the MHC/CD8 complex is considered as a specific immunological marker (2, 8). In this study, all of our patients with PM fulfilled the Bohan and Peter's criteria irrespective of the presence of inflammatory reactions in their muscle biopsies. All showed a marked sarcolemmal reactivity for MHC class I with or without CD8+ T cells, strongly supporting the importance of MHC/CD8 complex in the pathological diagnosis of PM.
In conclusion, although the inflammatory cell infiltrates can be observed in dysferlinopathy as well as in idiopathic inflammatory myopathies (PM and DM), there are significant differences in the types and patterns of infiltrating cells as seen in our study. The differences in the patterns of MHC class I expression and MAC deposits may also help to distinguish dysferlinopathy with inflammatory reactions and idiopathic inflammatory myopathy.
Figures and Tables
Fig. 1
Cellular composition of inflammatory infiltrates in polymyositis (patient 3, A-E), dermatomyositis (patient 10, F-J), and dysferlinopathy (patient 15, K-O). In polymyositis (PM), the inflammatory infiltrates are mainly observed in endomysial spaces, and CD8+ T cells are most abundant. In dermatomyositis (DM), there are two inflammatory infiltrates in perivascular areas. CD4+ T cells and macrophages are more frequent than others. In dysferlinopathy (DF), there are inflammatory infiltrates in perivascular areas. CD4+ T cells and macrophages are mainly observed as in DM. A few CD8+ T cell are also scattered in endomysial spaces, but they do not surround or invade a normal-appearing muscle fibers.
H&E, hematoxylin and eosin stain; CD8, immunostaining for CD8+ T cells; CD4, immunostaining for CD4+ T cells; CD20, immunostaining for B cells; CD68, immunostaining for macrophages.
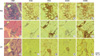
Fig. 2
Immunohistochemical localization of MHC class I in control, polymyositis, dermatomyositis, and dysferlinopathy. In control muscle (A), MHC class I reactivity is present on the endothelium, but staining of the sarcolemma is negative. In polymyositis (B-F) and dermatomyositis (G, H), the non-necrotic muscle fibers show marked sarcolemmal reactivity for MHC class I. In some patients with prominent inflammatory infiltrates (B, D), pronounced cytoplasmic staining is present in non-necrotic fiber as well as in necrotic fibers. In patients with dysferlinopathy (I-L), MHC class I is expressed in the extracellular matrix and endothelium, but not on the sarcolemma of muscle fibers.

Fig. 3
Immunohistochemical localization of MAC depositis. In PM (A) and DM (B), there are MAC depositis on vessel walls of arteriole and within necrotic muscle fibers. In dysferlinopathy (C, D), MAC deposits are found on the sarcolemma of nonnecrotic fibers as well as on vessel walls.
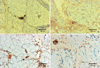
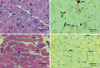
Fig. 4
In a patient with early stage DM (patient 10), MAC deposits are clearly observed on endomysial capillaries (A, B, arrows). In a patient with late stage DM (patient 12), there is no MAC deposit on capillaries (C, D, arrows).
References
1. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975. 292:403–407.
3. Briani C, Doria A, Sarzi-Puttini P, Dalakas MC. Update on idiopathic inflammatory myopathies. Autoimmunity. 2006. 39:161–170.

4. Gallardo E, Rojas-Garcia R, de Luna N, Pou A, Brown RH Jr, Illa I. Inflammation in dysferlin myopathy: immunohistochemical characterization of 13 patients. Neurology. 2001. 57:2136–2138.

5. Liu J, Aoki M, Illa I, Wu C, Fardeau M, Angelini C, Serrano C, Urtizberea JA, Hentati F, Ben Hamida M, Bohlega S, Culper EJ, Amato AA, Bossie K, Oeltjen J, Bejaoui K, McKenna-Yasek D, Hosler BA, Schurr E, Arahata K, de Jong PJ, Brown RH Jr. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nature Genet. 1998. 20:31–36.

6. Anderson LV, Davison K, Moss JA, Young C, Cullen MJ, Walsh J, Johnson MA, Bashir R, Britton S, Keers S, Argov Z, Mahjneh I, Fougerousse F, Beckmann JS, Bushby KM. Dysferlin is a plasma membrane protein and is expressed early in human development. Hum Mol Genet. 1999. 8:855–861.

7. Kim DS, Park KH, Nam SO, Lee CH, Park KJ. Significance of immunohistochemical study in patients with muscular dystrophy. J Korean Neurol Assoc. 2004. 22:613–622.
8. Dalakas MC. Inflammatory disorders of muscle: progress in polymyositis, dermatomyositis and inclusion body myositis. Curr Opin Neurol. 2004. 17:561–567.

9. Rowe DJ, Isenberg DA, McDougall J, Beverley PC. Characterization of polymyositis infiltrates using monoclonal antibodies to human leucocyte antigens. Clin Exp Immunol. 1981. 45:290–298.
10. Rowe D, Isenberg DA, Beverley PC. Monoclonal antibodies to human leucocyte antigens in polymyositis and muscular dystrophy. Clin Exp Immunol. 1983. 54:327–336.
11. Engel AG, Arahata K. Mononuclear cells in myopathies: quantitation of functionally distinct subsets, recognition of antigen-specific cell-mediated cytotoxicity in some diseases, and implications for the pathogenesis of the different inflammatory myopathies. Hum Pathol. 1986. 17:704–721.

12. Vianna MA, Borges CT, Borba EF, Caleiro MT, Bonfa E, Marie SK. Myositis in mixed connective tissue disease: a unique syndrome characterized by immunohistopathologic elements of both polymyositis and dermatomyositis. Arq Neuropsiquiatr. 2004. 62:923–934.

13. Brunn A, Schroder R, Deckert M. The inflammatory reaction pattern distinguishes primary dysferlinopathies from idiopathic inflammatory myopathies: an important role for the membrane attack complex. Acta Neuropathol. 2006. 112:325–332.

14. Bittner RE, Anderson LV, Burkhardt E, Bashir R, Vafiadaki E, Ivanova S, Raffelsberger T, Maerk I, Höger H, Jung M, Karbasiyan M, Storch M, Lassmann H, Moss JA, Davison K, Harrison R, Bushby KM, Reis A. Dysferlin deletion in SJL mice (SJL-Dysf) defines a natural model for limb girdle muscular dystrophy 2B. Nat Genet. 1999. 23:141–142.

15. Karpati G, Pouliot Y, Carpenter S. Expression of immunoreactive major histocompatibility complex products in human skeletal muscles. Ann Neurol. 1988. 23:64–72.

16. Emslie-Smith AM, Arahata K, Engel AG. Major histocompatibility complex class I antigen expression, immunolocalization of interferon subtypes, and T cell-mediated cytotoxicity in myopathies. Hum Pathol. 1989. 20:224–231.

17. Nyberg P, Wikman AL, Nennesmo I, Lundberg I. Increased expression of interleukin 1alpha and MHC class I in muscle tissue of patients with chronic, inactive polymyositis and dermatomyositis. J Rheumatol. 2000. 27:940–948.
18. Civatte M, Schleinitz N, Krammer P, Fernandez C, Guis S, Veit V, Pouget J, Harlé JR, Pellissier JF, Figarella-Branger D. Class I MHC detection as a diagnostic tool in noninformative muscle biopsies of patients suffering from dermatomyositis (DM). Neuropathol Appl Neurobiol. 2003. 29:546–552.

19. van der Pas J, Hengstman GJ, ter Laak HJ, Borm GF, van Engelen BG. Diagnostic value of MHC class I staining in idiopathic inflammatory myopathies. J Neurol Neurosurg Psychiatry. 2004. 75:136–139.
20. Bao SS, King NJ, dos Remedios CG. Elevated MHC class I and II antigens in cultured human embryonic myoblasts following stimulation with gamma-interferon. Immunol Cell Biol. 1990. 68:235–241.
21. Michaelis D, Goebels N, Hohlfeld R. Constitutive and cytokine-induced expression of human leukocyte antigens and cell adhesion molecules by human myotubes. Am J Pathol. 1993. 143:1142–1149.
22. Nagaraju K, Raben N, Loeffler L, Parker T, Rochon PJ, Lee E, Danning C, Wada R, Thompson C, Bahtiyar G, Craft J, Hooft Van Huijsduijnen R, Plotz P. Conditional up-regulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific autoantibodies. Proc Natl Acad Sci USA. 2000. 97:9209–9214.

23. Englund P, Nennesmo I, Klareskog L, Lundberg IE. Interleukin-1alpha expression in capillaries and major histocompatibility complex class I expression in type II muscle fibers from polymyositis and dermatomyositis patients: important pathogenic features independent of inflammatory cell clusters in muscle tissue. Arthritis Rheum. 2002. 46:1044–1055.
24. Englund P, Lindroos E, Nennesmo I, Klareskog L, Lundberg IE. Skeletal muscle fibers express major histocompatibility complex class II antigens independently of inflammatory infiltrates in inflammatory myopathies. Am J Pathol. 2001. 159:1263–1273.

25. Confalonieri P, Oliva L, Andreetta F, Lorenzoni R, Dassi P, Mariani E, Morandi L, Mora M, Cornelio F, Mantegazza R. Muscle inflammation and MHC class I up-regulation in muscular dystrophy with lack of dysferlin: an immunopathological study. J Neuroimmunol. 2003. 142:130–136.

26. Kostek CA, Dominov JA, Miller JB. Up-regulation of MHC class I expression accompanies but is not required for spontaneous myopathy in dysferlin-deficient SJL/J mice. Am J Pathol. 2002. 160:833–839.

27. Emslie-Smith AM, Engel AG. Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann Neurol. 1990. 27:343–356.

28. Kissel JT, Halterman RK, Rammohan KW, Mendell JR. The relationship of complement-mediated microvasculopathy to the histologic features and clinical duration of disease in dermatomyositis. Arch Neurol. 1991. 48:26–30.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download