Abstract
This study was aimed to evaluate the efficacy of a single administration of long-acting gonadotrophin-releasing hormone agonist (GnRHa) as compared with daily administrations of short-acting GnRHa in controlled ovarian hyperstimulation (COH) for in vitro fertilization and embryo transfer (IVF-ET) cycles. The mean dosage of recombinant follicle-stimulating hormone (rFSH) required for COH (2,354.5±244.2 vs. 2,012.5±626.1 IU) and the rFSH dosage per retrieved oocyte (336.7±230.4 vs. 292.1±540.4 IU) were significantly higher in the long-acting GnRHa group (N=22) than those in the short-acting GnRHa group (N=28) (p<0.05). However, the mean number of visit to the hospital that was required before ovum pick-up (3.3±0.5 vs. 22.2±2.0) and the frequency of injecting GnRHa and rFSH (12.8±1.2 vs. 33.5±3.5) were significantly decreased in the long-acting GnRHa group (p<0.0001). The clinical pregnancy rate, implantation rate, and early pregnancy loss rate were not significantly different between the 2 groups. So, we suggest that a single administration of long-acting GnRHa is a useful alternative for improving patient's convenience with clinical outcomes comparable to daily administrations of short-acting GnRHa in COH for IVF-ET cycles.
Gonadotrophin-releasing hormone (GnRH) analogues have been widely used for pituitary suppression in women undergoing controlled ovarian hyperstimulation (COH) for in vitro fertilization and embryo transfer (IVF-ET) cycle. The benefits of GnRH analogues are principally the reduction of serum luteinizing hormone (LH) concentration and the prevention of premature LH surge (1, 2). As a consequence of low LH concentration and prevention of LH surge, the incidence of cycle cancellation would be decreased and the convenience of both patient and physician would be improved (3, 4). Local ovarian androgen concentration is also reduced by the low level of LH, and this increased estrogen/androgen ratio improves oocyte development (5). Moreover, GnRH analogues contribute partly to improving endometrial receptivity and widening implantation window (6, 7). Overall, these benefits cause augmentation of mature oocytes and pregnancy rate in IVF-ET cycles (8-10).
Diverse GnRH analogues have been developed with different chemical structures, biological activity, half-life, and the routes of administration. Among the various COH protocols of combining GnRH analogues and gonadotrophins, the long protocol has been known as a more efficient method because of its adequate pituitary suppression and better success rate in comparison with the short protocol (11, 12). The factors taken into consideration when choosing the appropriate GnRH analogue for COH are: 1) the adequacy of pituitary suppression, 2) retrieval of high number of good oocytes, 3) length and cost of COH, as well as 4) outcome of pregnancy. In addition, the convenience of COH protocol is another important point to consider so as not to interfere with the patient's well-being. For example, multiple daily injections of the drug not only can cause frequent pain but also result in more time spent visiting hospital during COH.
Several studies were performed to compare the efficacy between long-acting GnRH agonist (GnRHa) and short-acting GnRHa (13-16). No significant differences in gonadotrophin doses, the duration of stimulation, the number of retrieved oocytes, fertilization rates as well as pregnancy rates between 2 types of GnRHa were reported. Recent meta-analysis, however, reported that long-acting GnRHa required more doses of gonadotrophin and longer duration of ovarian stimulation (17). In this study, we aim to evaluate the clinical outcome and patient's convenience of single administration of long-acting GnRHa (goserelin depot) as compared with multiple daily administrations of short-acting GnRHa (buserelin or leuprorelin).
A total of 50 patients were included in this prospective, case controlled study. Inclusion criteria were as follows: 1) aged between 25 and 40 yr; 2) infertility caused by tubal factor, endometriosis, male factor or unexplained factor; 3) serum basal FSH concentration <10 mIU/mL and serum basal estradiol <50 pg/mL just before starting COH; 4) presence of 2 functional ovaries and normal uterine cavity; 5) ≤3 previous IVF-ET attempts; and 6) no treatment of gonadotrophins within 3 month before this study.
All patients underwent pituitary desensitization by starting the administration of GnRHa on day 21 of the preceding IVF-ET cycle. In the long-acting GnRHa group (Group L), patients received 3.6 mg of subcutaneous goserelin acetate (Zoladex® depot, AstraZeneca Ltd., Kings Langley, U.K.). In the short-acting GnRHa group (Group S), patients were subcutaneously administered 0.5 mg of buserelin acetate (Superfact®, Hoechst AG, Frankfurt, Germany) or leuprolide acetate (Lucrin®, Labratories Abbot France, St. Remy Sur Avre, France) daily at hospital before human chorionic gonadotrophin (hCG) administration. Ovarian stimulation was started from day 2 or 3 of the menstrual cycle with daily self-administration (100-250 IU) of human recombinant follicle-stimulating hormone (rFSH) and continued on the day of hCG injection. The 10,000 IU of hCG (Pregnyl®, Organon, Oss, Netherlands) was administered when the leading follicle was larger than 18 mm in mean diameter, and at least 2 follicles were larger than 16 mm. Oocytes were retrieved 36 hr after hCG injection using a 17-gauge aspiration needle with transvaginal ultrasound guidance.
The grade of oocyte and embryo (day 2-5) was determined according to the criteria of Veeck by 3 investigators in a blinded manner as previously described (18). Oocytes were preincubated in the medium at 37℃ with 6% CO2 for 4-6 hr and inseminated by conventional IVF or intracytoplasmic sperm injection (ICSI). The fertilization was confirmed when 2 polar bodies and 2 pronuclei were observed at 16-20 hr after insemination. For fertilization and embryo culture, we used the sequential media system, commercially produced by Vitrolife (GIII series™, Vitrolife, Kungsbacka, Sweden) according to the manufacturer's guidance.
Two to four embryos were transferred into the uterine cavity using transfer catheter (Sydney IVF® Embryo transfer set, Cook Ireland Ltd, Limerick, Ireland), and surplus embryos were cryopreserved. There was no cancellation of embryo transfer in this study. The luteal phase support was initiated from the day before oocyte retrieval and continued up to 8 gestational weeks by progesterone in oil (Progest®, Samil Pharm, Seoul, Korea). The clinical pregnancy was confirmed by the fetal heart activity using a transvaginal sonography 4 weeks after oocyte retrieval.
The statistical analysis using GraphPad InStat (GraphPad Software, Inc., San Diego, U.S.A.) was performed with the student's t-test (2-tailed) or the Mann-Whitney U test (2-tailed) for comparison of means and the chi-square test and Fisher's exact test for proportions. p<0.05 was considered statistically significant.
The group L and S had similar clinical features with respect to age, duration of infertility, basal serum estradiol concentration, and the number of previous IVF attempts (Table 1).
The serum estradiol level on hCG administration day was significantly lower in group L (1,935.0±1,524.5 pg/mL vs. 2,630.1±1,210.0 pg/mL, p<0.05). The group L required more gonadotrophins for COH. A total dosage of rFSH for COH (2,354.5±244.2 IU vs. 2,012.5±626.1 IU, p<0.05) and a mean dosage of rFSH per retrieved oocyte (336.7±228.3 IU vs. 296.7±540.4 IU, p<0.05) were significantly increased in group L. The duration required for ovarian stimulation, the number of retrieved oocytes and produced embryos, and the number of transferred embryos and transferred good embryos were not significantly different in the 2 groups. Moreover, the rate of early pregnancy loss was not different between the 2 groups (5.9% vs. 6.3%, p=0.965). With respect to the clinical outcomes, clinical pregnancy rate (72.7% vs. 53.6%, p=0.275) and implantation rate (31.3% vs. 20.0%, p=0.088) were higher in group L than those in group S, but these differences were not statistically significant (Table 2). Table 3 showed that there was no significant difference of oocyte and embryo quality between the 2 groups. In addition, the fertilization rate was also similar in the 2 groups (75.3% vs. 79.2%, p=0.343).
In Table 4, the patient's convenience and the cost of medication for COH were summarized. The frequency of visiting hospital (3.3±0.5 vs. 22.2±2.0, p<0.0001) and the total number of injections with gonadotrophin and GnRHa (12.8±1.2 vs. 33.5±3.5, p<0.0001) were highly reduced in group L. The total cost of gonadotrophin and GnRHa consumed for COH was increased in group L (851.1±233.0 USD vs. 1,174.3±86.2 USD, p<0.0001).
The long protocol using GnRHa and gonadotrophin has been well known to be useful in COH of IVF-ET (11, 12). In spite of the benefits of long protocol, multiple daily injections of GnRHa are bothersome for women undergoing COH, including pain, psychosocial distress, and frequent hospital visits. Moreover, Wasser et al. (19) suggested that psychosocial distress contributes significantly to the etiology of some forms of infertility. So, simplifying the process of stimulation cycle is valuable to reduce patient's inconvenience and stress. Recently, self-injecting pen type of rFSH has been widely used and it has been shown to improve the patient's convenience (20). In comparison with daily administration of short-acting GnRHa, a single administration of long-acting GnRHa can append these advantages by reducing the number of injections in COH (13). Taken together, a single administration of long-acting GnRHa in combination with self-injecting pen type of rFSH can significantly improve the patient's convenience and comfort. In our data, the frequency of hospital visits and the number of injections (GnRHa and gonadotrophins) were significantly decreased by using long-acting GnRHa. In addition, the reduced number of visiting hospital can significantly decrease the cost for medical service.
Along with previous reports (13, 14, 16, 21, 23), this study demonstrated that a single administration of long-acting GnRHa does not prolong the duration of ovarian stimulation. However, recent meta-analytical study concluded that the duration of ovarian stimulation was significantly increased in the long-acting GnRHa group than in short-acting GnRHa group (17). It is noteworthy to note that the difference of duration of gonadotrophin treatment was less than 1 day. So, it would be concluded that the difference of duration in ovarian stimulation was not clinically important in both groups.
Also, the total dose of gonadotrophin required for COH is another important point for both physicians and the patients to consider when choosing the type of GnRHa and protocol because it can change the cost of COH. Numerous studies using human menopausal gonadotrophin reported that there was a controversy in the augmentation of total gonadotrophin consumption in the long-acting GnRHa group as compared with the short-acting GnRHa group (13-16, 21, 22). This controversy was also found in other studies using highly purified urinary FSH (24, 25). In our study, the total amount of rFSH required for COH significantly increased in the long-acting GnRHa group as compared with the short-acting GnRHa group, and this increased gonadotrophin consumption consequently elevated the cost of COH in the long-acting GnRHa group. From this study, however, we may suggest a solution to compensate the increased cost of gonadotrophin with reduced number of hospital visits. As shown in its. As shown in Table 4, the cost for COH was significantly increased in the long-acting GnRHa group, because the total dose of rFSH increased and the long-acting form of GnRHa was more expensive than short-acting form. To the best of our knowledge, there has been no report to compare the clinical efficacy of long-acting GnRHa with that of short-acting GnRHa in combination with self-injecting pen type rFSH. In the long protocol, the combination of long-acting GnRHa with self-injecting pen type rFSH can reduce the number and the cost of hospital visits to have an injection of GnRHa and gonadotrophins. Consequently, the total cost of COH using long-acting GnRHa became similar to the cost using short-acting GnRHa. Moreover, the reduced frequency of injection and hospital visits can improve the patient's quality of life, including convenience, less pain, and psychosocial comfort.
No matter what benefits the use of long-acting GnRHa may have as compared with short-acting GnRHa, the outcome of pregnancy is the most valuable factor in IVF-ET cycles. So, the use of long-acting GnRHa must have no detrimental effect on pregnancy outcome. Most of previous studies showed that there was no significant difference in pregnancy rate between long-acting and short-acting GnRHa (14, 16, 21-24). In this study, the clinical pregnancy rate as well as implantation rate was about 1.5 fold higher in the long-acting GnRHa group than in the short-acting GnRHa group, but this difference had no statistical significance because of the small sample size. In addition, we found that the quality of retrieved oocytes and produced embryos was not significantly different between the 2 groups.
In conclusion, our study demonstrated that a single administration of long-acting GnRHa in combination with self-injecting pen type of gonadotrophin can improve the patient's quality of life without a significant difference in clinical outcomes and total cost for COH of IVF-ET cycles. So, we would suggest that a single administration of long-acting GnRHa as a good alternative for improving patient's convenience and psychosocial comfort during COH of long protocol.
Figures and Tables
Table 2
Ovarian responses and clinical outcomes in the 2 groups
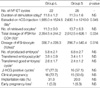
*Values are mean±SD; †β-hCG ≥5 mIU/mL; ‡No fetal heart activity in β-hCG positive cycles.
IVF-ET, in vitro fertilization and embryo transfer; hCG, human chorionic gonadotrophin; rFSH, recombinant follicle-stimulating hormone; COH, controlled ovarian hyperstimulation.
Values in parentheses are percentage.
ACKNOWLEDGMENTS
The authors thank Yoon Jeong Lee and Mi Jin Ryu at the Laboratory of Reproductive Medicine, Samsung Women's Hospital for their critical comments on the manuscript.
References
1. Loumaye E. The control of endogenous secretion of LH by gonadotrophin-releasing hormone agonists during ovarian hyperstimulation for in-vitro fertilization and embryo transfer. Hum Reprod. 1990. 5:357–376.

2. Smitz J, Van Den Abbeel E, Camus M, Devroey P, Tournaye H, Van Steirteghem AC. The effect of gonadotrophin-releasing hormone (GnRH) agonist in the follicular phase on in vitro fertilization outcome in normo-ovulatory women. Hum Reprod. 1992. 7:1098–1102.
3. Tummon IS, Daniel SA, Kaplan BR, Nisker JA, Yuzpe AA. Randomized, prospective comparison of luteal leuprolide acetate and gonadotropins versus clomiphene citrate and gonadotropin in 408 first cycles of in vitro fertilization. Fertil Steril. 1992. 58:563–568.
4. Dimitry ES, Bates SA, Oskarsson T, Margara R, Winston RM. Programming in vitro fertilization for a 5- or 3-day week. Fertil Steril. 1991. 55:934–938.

5. Polan ML, Daniele A, Russell JB, DeCherney AH. Ovulation induction with human menopausal gonadotropin compared to human urinary follicle-stimulating hormone results in a significant shift in follicular fluid androgen levels without discernible differences in granulosa-luteal cell function. J Clin Endocrinol Metab. 1986. 63:1284–1291.

6. Testart J, Forman R, Belaisch-Allart J, Volante M, Hazout A, Strubb N, Frydman R. Embryo quality and uterine receptivity in in-vitro fertilization cycle with or without agonists of gonadotrophin-releasing hormone. Hum Reprod. 1989. 4:198–201.
7. Tur-Kaspa I, Confino E, Dudkiewicz AB, Myers SA, Friberg J, Gleicher N. Ovarian stimulation protocol for in-vitro fertilization with gonadotropin-releasing hormone agonist widens the implantation window. Fertil Steril. 1990. 53:859–864.
8. Frydman R, Belaisch-Allart J, Parneix I, Forman R, Hazout A, Testart J. Comparison between flare up and down regulation effects of luteinizing hormone-releasing hormone agonist in an in vitro fertilization program. Fertil Steril. 1988. 50:471–475.
9. Abdalla HI, Ahuja KK, Leonard T, Morris NN, Honour JW, Jacobs HS. Comparative trial of luteinizing hormone-releasing hormone analog/human menopausal gonadotropin and clomiphene citrate/human menopausal gonadotropin in assisted conception program. Fertil Steril. 1990. 53:473–478.
10. Hughes EG, Fedorkow DM, Daya S, Sagle MA, Van de Kooppel P, Collins JA. The routine use of gonadotrophin-releasing hormone agonists prior to in-vitro fertilization and gamete intra-fallopian transfer: a meta-analysis of randomized controlled trials. Fertil Steril. 1992. 58:888–896.
11. Ron-El R, Herman A, Golan A, van der Ven H, Caspi E, Diedrich K. The comparison of early follicular and midluteal administration of long acting gonadotropin-releasing hormone agonist. Fertil Steril. 1990. 54:233–237.
12. Tan SL, Kingsland C, Campbell S, Mills C, Bradfield J, Alexander N, Yovich J, Jacobs HS. The long protocol of administration of gonadotropin-releasing hormone agonist is superior to the short protocol for ovarian stimulation for in vitro fertilisation. Fertil Steril. 1992. 57:810–814.
13. Oyesanya OA, Teo SK, Quah E, Abdurazak N, Lee FY, Cheng WC. Pituitary down-regulation prior to in-vitro fertilization and embryo transfer: a comparison between a single dose of Zoladex depot and multiple daily doses of Suprefact. Hum Reprod. 1995. 10:1042–1044.
14. Hsieh Y, Tsai H, Chang C, Lo H. Comparison of a single half-dose, long-acting form of gonadotropin-releasing hormone analog (GnRH-a) and a short-acting form of GnRH-a for pituitary suppression in a controlled ovarian hyperstimulation program. Fertil Steril. 2000. 73:817–820.

15. El-Nemr A, Bhide M, Khalifa Y, Al-Mizyen E, Gillott C, Lower AM, Al-Shawaf T, Grudzinskas JG. Clinical evaluation of three different gonadotrophin-releasing hormone analogues in an IVF programme: a prospective study. Eur J Obstet Gynecol Reprod Biol. 2002. 103:140–145.

16. Geber S, Sales L, Sampaio MA. Comparison between a single dose of goserelin (Depot) and multiple daily doses of leuprolide acetate for pituitary suppression in IVF treatment: a clinical endocrinological study of the ovarian response. J Assist Reprod Genet. 2002. 19:313–318.
17. Albuquerque LE, Saconato H, Maciel MC, Baracat EC, Freitas V. Depot versus daily administration of GnRH agonist protocols for pituitary desensitization in assisted reproduction cycles: a Cochrane review. Hum Reprod. 2003. 18:2008–2017.

18. Cheon KW, Byun HK, Yang KM, Song IO, Choi KH, Yoo KJ. Efficacy of recombinant human follicle-stimulating hormone in improving oocyte quality in assisted reproductive techniques. J Reprod Med. 2004. 49:733–738.
19. Wasser SK, Sewall G, Soules MR. Psychosocial stress as a cause of infertility. Fertil Steril. 1993. 59:685–689.
20. Pang SC. A pen injection device for self-administration of recombinant follicle-stimulating hormone for fertility treatments. Expert Rev Med Devices. 2005. 2:27–32.

21. Tapanainen J, Hovatta O, Juntunen K, Martikainen H, Ratsula K, Tulppala M, Tuomivaara L. Subcutaneous goserelin versus intranasal buserelin for pituitary down-regulation in patients undergoing IVF: a randomized comparative study. Hum Reprod. 1993. 8:2052–2055.
22. Tsai HD, Chen CM, Lo HY, Chang CC. Subcutaneous low dose leuprolide acetate depot versus leuprolide acetate for women undergoing ovarian stimulation for in-vitro fertilization. Hum Reprod. 1995. 10:2909–2912.

23. Dada T, Salha O, Baillie HS, Sharma V. A comparison of three gonadotrophin-releasing hormone analogues in an in-vitro fertilization programme: a prospective randomized study. Hum Reprod. 1999. 14:288–293.

24. Dal Prato L, Borini A, Trevisi MR, Bonu MA, Sereni E, Flamigni C. Effect of reduced dose of triptorelin at the start of ovarian stimulation on the outcome of IVF: a randomized study. Hum Reprod. 2001. 16:1409–1414.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download