Abstract
Lymph node metastasis is an important prognostic factor in gastric cancer. Vascular endothelial growth factor-D (VEGF-D) is a lymphangiogenic growth factor that activates VEGF receptor (VEGFR)-3, a receptor expressed in the lymphatic endothelium. We investigated the clinical value of VEGF-D expression and VEGFR-3 positive vessel density in gastric carcinoma with regard to lymphangiogenesis. Immunohistochemical staining was used to determine the expression of VEGF-D and VEGFR-3 in specimens from 104 cases of resected gastric cancer. VEGF-D expression was observed in 62.5% of the gastric cancers and in 9.6% of the non-neoplastic gastric tissue. The VEGFR-3-positive vessel density was significantly greater in the VEGFD positive group than the negative group. VEGF-D expression was significantly associated with lymph node metastasis, increased serum CEA levels, and the non-signet ring cell type. The VEGFR-3-positive vessel density was correlated with tumor size, lymphatic invasion, and lymph node metastasis. The VEGF-D expression and high VEGFR-3-positive vessel density were significant poor prognostic factors for relapse-free survival. These results suggest that VEGF-D and VEGFR-3-positive vessel density are potential molecular markers that predict lymphatic involvement in gastric carcinoma.
Gastric cancer is one of the leading causes of death in the world. Metastasis to the regional lymph node is an indicator of tumor progression as well as an important prognostic factor in gastric cancer. Recent evidence suggests that tumor lymphangiogenesis promotes lymphatic metastasis (1-3). However, little is known about the mechanism of lymphangiogenesis in gastric carcinoma.
Vascular endothelial growth factor (VEGF)-C and VEGF-D are the best-characterized lymphangiogenic growth factors. These growth factors stimulate lymphangiogenesis by activating VEGF receptor (VEGFR)-3, also known as Flt (fms-like tyrosine kinase)-4, a receptor which is expressed in the lymphatic endothelium (4-7). VEGFR-3 was once thought to be a marker of lymphatic endothelial cells because it is mainly expressed in the lymphatic endothelium of adult tissue (8); however, VEGFR-3 has also been detected in blood vessels within tumors and wounds that are healing (9, 10).
Several studies have correlated VEGF-D expression with lymph node metastasis in a variety of cancers including colorectal (11), breast (12), pancreatic (13), ovarian (14), and endometrial (15). Furthermore, a high VEGFR-3-positive vessel density has been correlated with poor prognosis in breast cancer (16) and non-small cell lung cancer (17).
The role of VEGF-D and VEGFR-3 in gastric carcinoma has not been fully determined. Recently, Jutter et al. reported that VEGF-D and VEGFR-3 are novel independent prognostic marker molecules for reduced survival after the curative resection of gastric adenocarcinoma (18). The goal of our study was to investigate the clinical value of VEGF-D expression and VEGFR-3-positive vessel density in gastric carcinoma with regard to lymphangiogenesis.
This study comprised 104 patients who underwent surgical resection for gastric adenocarcinoma at Hanyang University Guri Hospital between April 2000 and November 2003. Of those, 84 patients had advanced gastric cancers and 20 patients had early gastric cancers. Well-documented clinical data were collected from all patients. Information concerning the date of initial diagnosis, clinical characteristics, relapse, and death were retrospectively obtained. In addition, adjacent non-neoplastic stomach tissue samples as confirmed by Hematoxylin and Eosin staining were used as controls. This study was approved by the institutional review board of Hanyang University Guri Hospital.
The avidin-biotin complex (ABC) method was used for immunostaining. Formalin-fixed, paraffin-embedded tissue blocks were sectioned at a 4-µm thickness. The tissue sections were deparaffinized by three, 10-min incubations in xylene and then rehydrated in serial graded alcohol. For antigen retrieval, the sections were heated in a microwave oven for 10 min in 10 mM/L sodium citrate buffer (pH 6). Endogenous peroxidase activity was eliminated by preincubation in 3% hydrogen peroxide and 10% methanol for 15 min followed by three washes in phosphate-buffered saline. All slides were pre-incubated at 37℃ for 20 min with two drops of normal blocking solution (goat serum, 100 µL/slide). The slides were then incubated with either a goat polyclonal anti-VEGF-D antibody (R&D Systems, Minneapolis, MN) at a 1:100 dilution overnight at 4℃ or a rabbit polyclonal anti-VEGFR-3 antibody (Zymed Laboratories, San Francisco, CA, U.S.A.) at a 1:200 dilution for 2 hr at room temperature. Biotinylated secondary antibody was added to each slide and incubated for 30 min at 37℃. The slides were then treated with the avidin-biotinylated peroxidase complex (Immunotech, Cedex, France) for an additional 30 min at room temperature. 3, 3'-diaminobenzidine tetrahydrochloride (Immunotech, Cedex, France) was used for color development. Finally, the sections were counterstained with hematoxylin.
All slides were coded and evaluated by two experienced pathologists without knowledge of patient identity or clinical status. Each experiment was performed twice independently. In the discrepant cases, two pathologists reviewed the cases together and reached a consensus. The VEGF-D staining intensity was scored as 0 (negative), 1 (weak), 2 (medium), and 3 (strong). The extent of staining was scored according to the percentage of areas with positive VEGF-D staining as follows: 0 (0%), 1 (1 to 25%), 2 (26 to 50%), 3 (51 to 75%), and 4 (76 to 100%). The final staining score was derived from the sum of the intensity score and the extent score. Tumors with a final staining score of ≥5 were considered as positive for VEGF-D expression.
The VEGFR-3-positive vessel density was assessed according to the method described by Weidner et al. (19). Microvessel counting was performed twice. Each slide was first scanned at 100× magnification to determine three "hot spots" defined as areas with the maximum number of VEGFR-3-positive vessels. The VEGFR-3-positive vessel density was determined by counting all the immunostained vessels at a 200× magnification and the mean number of positive vessels was calculated in the three selected areas for each case.
The Pearson chi-square (χ2) test was performed to determine the correlation between VEGF-D expression and various clinicopathological factors. The Mann-Whitney U test and Kruskal-Wallis test were used to examine the association of VEGFR-3-positive vessel density. Survival curves were calculated using the Kaplan-Meier method and compared with other prognostic variables using the log-rank test. A stepwise Cox's regression analysis was performed to identify prognostic factors for survival. In all tests, p<0.05 was considered statistically significant. All statistical analyses were performed using SPSS 10® statistical software.
Our study included 71 male (68.3%) and 33 female (31.7 %) patients, with a median age of 59 yr (range, 25-79 yr). All tumors were staged using the AJCC classification. Forty-three patients (41.4%) were classified as stage I, 11 patients (10.6%) as stage II, 20 patients (19.2%) as stage III, and 30 patients (28.8%) as stage IV. R0 resection was done in 95 patients, R1 resection in 4, and R2 resection in 5. Forty-one patients received intravenous systemic chemotherapy after surgery (17 patients: cisplatin+5-fluorouracil (5-FU), 16 patients: heptaplatin +5-FU, and 8 patients: paclitaxel+cisplatin+5-FU) and 56 patients received 5-fluorouracil orally. The median follow-up period was 35.9 months (range: 2.1-70.5 months). Thirty-five patients had relapsed by the time of last follow-up and thirty-five patients died. The most common cause of death was disease progression (30 patients), while other causes of death included respiratory failure (1 patient), bowel infarction (1 patient), liver cirrhosis (1 patient), malnutrition (1 patient), and septic shock (1 patient).
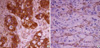
A granular pattern of VEGF-D staining was observed within the cytoplasm of malignant gastric epithelium (Fig. 1). VEGF-D expression was observed in 62.5% of gastric cancers and in 9.6% of non-neoplastic gastric tissues (p<0.001). VEGF-D expression was significantly associated with lymph node metastasis (p<0.05), increased serum carcino embryonic antigen (CEA) levels (p<0.05), and the non-signet ring cell type (p<0.001, Table 1). However, we found no significant differences in other clinicopathological parameters such as age, sex, primary tumor size, grade, lymphatic and venous invasion, depth of tumor invasion (T stage), distant metastasis, intravenous systemic chemotherapy, curative respectability (R0/R1, R2), and stage between the VEGF-D-positive and -negative groups.
We observed VEGFR-3 expression on endothelial cells. Most of the VEGFR-3-stained vessels were thin-walled and did not contain red blood cells (Fig. 1). The VEGFR-3 positive vessel density was significantly greater in gastric carcinomas (median, 49.5; range, 0-205) than in non-neoplastic tissues (26.0; 0-98). Furthermore, the VEGFR-3-positive vessel densities were 60.0 (10-205) and 36 (0-100) in the VEGF-D positive and negative groups, respectively (p<0.001, Fig. 2). The VEGFR-3 positive vessel density was significantly correlated with primary tumor size (p<0.05), lymphatic invasion (p<0.05), and lymph node metastasis (p<0.05, Table 1). However, there was no significant difference in other clinicopathological parameters such as age, sex, pathologic type, grade, venous invasion, depth of tumor invasion, and distant metastasis.
To evaluate the correlation between VEGFR-3-positive vessel density and patient survival, the patients were divided into two groups: low (<50) and high VEGFR-3-positive vessel density (≥50). The cutoff value for each group was determined by the median VEGFR-3-positive vessel density. Univariate analysis showed that tumor size (p<0.001), lymphatic invasion (p<0.05), venous invasion (p<0.05), T (p< 0.001), N (p<0.001), M (p<0.001), and curative resectability (p<0.001) were significant factors for overall survival. However, VEGF-D expression (p>0.05) and high VEGFR-3-positive vessel density (p>0.05) did not influence overall survival. Patients with VEGF-D expression had a significantly shorter relapse-free survival time compared to patients who were VEGF-D-negative (5 yr relapse-free survival rate 55.8% vs. 67.6%, p<0.05, Fig. 3). Furthermore, the high VEGFR-3-positive vessel density group showed a significantly worse relapse-free survival when compared to the low positive vessel density group (5 yr relapse-free survival rate 48.9% vs. 74.9%, p=0.037, Fig. 3). In addition, increased serum CEA levels (p<0.05), tumor size (p<0.001), lymphatic invasion (p<0.001), venous invasion (p<0.05), T-category (p<0.001), N-category (p<0.001), M-category (p<0.001), and curative resectability (p<0.001) were significant prognostic factors for relapse-free survival.
In the multivariate analysis, TNM stage and curative resectability were the independent prognostic factors for disease-free survival and overall survival. The expression of VEGF-D and high VEGFR-3-positive vessel density did not influence survival.
The known lymphangiogenic growth factors VEGF-C and VEGF-D are structurally similar secreted glycoproteins. These growth factors induce lymphangiogenesis and angiogenesis in tissues and tumors by activating VEGFR-3, a receptor expressed in the lymphatic endothelium of adults, and VEGFR-2, a receptor expressed in the endothelium of blood vessels. VEGF-C and VEGF-D are synthesized as proproteins. Subsequently, the propeptides can be proteolytically removed to generate mature forms consisting of VEGF homology domain dimers. The full-length forms of VEGF that are initially secreted to activate VEGFR-3 but not VEGFR-2. However, after proteolytic processing, both VEGF-C and VEGF-D bind VEGFR-2 and VEGFR-3 with high affinity (20, 21).
Previous work has associated VEGF-C and VEGF-D expression with cancer progression. In a mouse tumor model, the expression of VEGF-D in tumor cells stimulated the formation of intratumoral lymphatics, angiogenesis, tumor growth, and metastatic spread of tumor cells via the lymphatic vessels. This VEGF-D-induced lymphatic spread could be blocked by an antibody specific for VEGF-D (1). In addition, a recent study reported that VEGF-C and VEGF-D induced lymphangiogenesis in experimental gastric tumors by inducing VEGFR-3 expression (22). Recently, a clinicopathological study with 91 cases of resected primary gastric adenocarcinoma showed that VEGF-D correlated with lymphatic metastasis and decreased survival and that VEGF-D and VEGFR-3 were independent factors associated with poor survival (18).
However, there are only a few reports regarding the expression of VEGF-D and VEGFR-3 in gastric cancer. And the association between VEGF-D/VEGFR-3 and lymph node metastasis remains even less understood. Some reports have shown a significant correlation of VEGF-D with lymphatic invasion or lymph node metastasis (23-25) in gastric cancer, whereas others have found no relationship between them (26, 27). Furthermore, Yonemura et al. reported that the number of VEGFR-3-positive vessels was closely related to lymphatic invasion and lymph node metastasis in primary gastric cancer (28) and another study showed that the expression of VEGFR-3 was significantly greater in the node-positive group (26). However, there was also a study that did not find such correlation (25).
In our study, we found that the VEGF-D expression rate and VEGFR-3-positive vessel density were significantly greater in gastric carcinoma tissue than in non-neoplastic tissue and that VEGF-D expression was associated with VEGFR-3-positive vessel density. Our work also showed that VEGF-D expression was significantly related to lymph node metastasis and that VEGFR-3-positive vessel density was significantly correlated with lymphatic invasion, and lymph node metastasis. These results suggest that VEGF-D plays an important role in lymphangiogenesis and lymph node metastasis through VEGFR-3 in gastric adenocarcinoma.
In our study, VEGF-D expression and high VEGFR-3-positive vessel density were significant prognostic factors for relapse-free survival. However, in our results, VEGF-D expression and high VEGFR-3-positive vessel density did not influence overall survival. The discrepancies in the results between our work and the previous study (18) may be due to differences in the gastric cancer operation method and the relatively short follow-up duration in our study. Although the p value was not statistically significant, the survival curves according to VEGF-D expression and VEGFR-3-positive vessel density separated as time passed. Therefore, a long-term follow-up is needed to confirm whether VEGF-D expression and/or VEGFR-3-positive vessel density are significant prognostic factors.
A previous study reported that VEGF-D expression was lower in undifferentiated cancer than in differentiated gastric cancer (23). Although VEGFR-3-positive vessel density was not significantly different between the two groups, we also found that VEGF-D expression was lower in carcinomas of signet ring cell type (39.5%) than in non-signet ring cell type (75.8%). However, signet ring cell type gastric carcinomas usually have high rates of lymph node metastasis as well as poor prognosis. Although we could not find the reason for these results, we thought that these contradictory findings might be explained by the low binding affinity of the antibody to signet ring cell carcinoma via an unexplained mechanism or by the existence of other cytokines that could promote lymphangiogenesis. Further studies are needed to elucidate the cause of this apparent contradiction.
Lymphangiogenesis is one of the important mechanisms which contribute to the progression of cancer. Because inhibitors that block the VEGF-C/VEGF-D/VEGFR-3 signaling pathway might potentially block lymphangiogenous metastasis, the VEGF-C/VEGF-D/VEGFR-3 interaction has been extensively investigated as a possible target for cancer treatment. Potential antilymphangiogenic therapeutics include soluble versions of VEGFR-3 that bind VEGF-C and VEGF-D, thereby inhibiting activation of endogenous VEGFR-3, neutralizing monoclonal antibodies to VEGF-C and VEGF-D that inhibit binding to both VEGFR-2 and VEGFR-3, monoclonal antibodies to VEGFR-3, and small molecules that inhibit VEGFR-3 tyrosine kinase or downstream signaling molecules (21). Some of these agents might provide added benefit for patients as new molecular targeted therapies.
Our study had several limitations. First, immunohistochemical staining was the only method used to determine VEGF-D and VEGFR-3 expression. More accurate results could be obtained by combining the immunohistochemistry results with data from reverse transcription polymerase chain reactions and western blots. Second, the follow-up duration in this study was short. Third, the clinical data, including relapse or survival data, was analyzed retrospectively. Lastly, a relatively small number of patients were examined in this study.
Nevertheless, our results suggest that VEGF-D and VEGFR-3-positive vessel density are potential molecular markers that predict lymphatic involvement in gastric carcinoma. These potential markers could be candidates for a new area of molecular therapeutic targeting for gastric cancer.
Figures and Tables
Fig. 1
Immunohistochemical staining of a gastric carcinoma. Each photograph shows representative tissue that is positive for VEGF-D (A) and VEGFR-3 (B) (×400).
References
1. Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001. 7:186–191.

2. Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001. 20:672–682.

3. Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001. 7:192–198.

4. Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996. 15:290–298.

5. Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997. 276:1423–1425.

6. Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci USA. 1998. 95:548–553.

7. Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV, Kubo H, Thurston G, McDonald DM, Achen MG, Stacker SA, Alitalo K. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001. 20:1223–1231.

8. Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA. 1995. 92:3566–3570.

9. Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000. 60:203–212.
10. Paavonen K, Puolakkainen P, Jussila L, Jahkola T, Alitalo K. Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am J Pathol. 2000. 156:1499–1504.

11. White JD, Hewett PW, Kosuge D, McCulloch T, Enholm BC, Carmichael J, Murray JC. Vascular endothelial growth factor-D expression is an independent prognostic marker for survival in colorectal carcinoma. Cancer Res. 2002. 62:1669–1675.
12. Nakamura Y, Yasuoka H, Tsujimoto M, Yang Q, Imabun S, Nakahara M, Nakao K, Nakamura M, Mori I, Kakudo K. Prognostic significance of vascular endothelial growth factor D in breast carcinoma with long-term follow-up. Clin Cancer Res. 2003. 9:716–721.
13. Kurahara H, Takao S, Maemura K, Shinchi H, Natsugoe S, Aikou T. Impact of vascular endothelial growth factor-C and -D expression in human pancreatic cancer: its relationship to lymph node metastasis. Clin Cancer Res. 2004. 10:8413–8420.
14. Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, Emoto M, Umemoto M, Sakamoto T, Sato S, Mizunuma H, Smith SK. Vascular endothelial growth factor-D is an independent prognostic factor in epithelial ovarian carcinoma. Br J Cancer. 2003. 88:237–244.

15. Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, Emoto M, Sakamoto A, Sakamoto T, Maruyama H, Sato S, Mizunuma H, Smith SK. Expression of vascular endothelial growth factor (VEGF)-D and its receptor, VEGF receptor 3, as a prognostic factor in endometrial carcinoma. Clin Cancer Res. 2003. 9:1361–1369.
16. Nakamura Y, Yasuoka H, Tsujimoto M, Yang Q, Imabun S, Nakahara M, Nakao K, Nakamura M, Mori I, Kakudo K. Flt-4-positive vessel density correlates with vascular endothelial growth factor-D expression, nodal status, and prognosis in breast cancer. Clin Cancer Res. 2003. 9:5313–5317.
17. Chen F, Takenaka K, Ogawa E, Yanagihara K, Otake Y, Wada H, Tanaka F. Flt-4-positive endothelial cell density and its clinical significance in non-small cell lung cancer. Clin Cancer Res. 2004. 10:8548–8553.

18. Jüttner S, Wissmann C, Jöns T, Vieth M, Hertel J, Gretschel S, Schlag PM, Kemmner W, Höcker M. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol. 2006. 24:228–240.

19. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N Engl J Med. 1991. 324:1–8.
20. Achen MG, McColl BK, Stacker SA. Focus on lymphangiogenesis in tumor metastasis. Cancer Cell. 2005. 7:121–127.

21. Achen MG, Mann GB, Stacker SA. Targeting lymphangiogenesis to prevent tumour metastasis. Br J Cancer. 2006. 94:1355–1360.

22. Yonemura Y, Endo Y, Tabata K, Kawamura T, Yun HY, Bandou E, Sasaki T, Miura M. Role of VEGF-C and VEGF-D in lymphangiogenesis in gastric cancer. Int J Clin Oncol. 2005. 10:318–327.

23. Ishikawa M, Kitayama J, Kazama S, Nagawa H. Expression of vascular endothelial growth factor C and D (VEGF-C and -D) is an important risk factor for lymphatic metastasis in undifferentiated early gastric carcinoma. Jpn J Clin Oncol. 2003. 33:21–27.

24. Shida A, Fujioka S, Ishibashi Y, Kobayashi K, Nimura H, Mitsumori N, Suzuki Y, Kawakami M, Urashima M, Yanaga K. Prognostic significance of vascular endothelial growth factor D in gastric carcinoma. World J Surg. 2005. 29:1600–1607.

25. Shida A, Fujioka S, Kobayashi K, Ishibashi Y, Nimura H, Mitsumori N, Yanaga K. Expression of vascular endothelial growth factor (VEGF)-C and -D in gastric carcinoma. Int J Clin Oncol. 2006. 11:38–43.

26. Kitadai Y, Kodama M, Cho S, Kuroda T, Ochiumi T, Kimura S, Tanaka S, Matsumura S, Yasui W, Chayama K. Quantitative analysis of lymphangiogenic markers for predicting metastasis of human gastric carcinoma to lymph nodes. Int J Cancer. 2005. 115:388–392.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download