Abstract
Very late stent thrombosis (VLST) after implantation of drug-eluting stent is rare, but very fatal complication after percutaneous coronary intervention. We report a case of VLST of a sirolimus-eluting Cypher™ stent (Cordis, Johnson and Johnson) presenting as acute ST elevation myocardial infarction at 26 months after deployment with continued combined dual antiplatelet medication of aspirin and clopidogrel. The patient did not show anti-platelet resistance.
Although drug-eluting stents (DES) have dramatically reduced the rate of restenosis, concerns about the risk of stent thrombosis (ST) has been raise. ST remains as a major cause of death after DES implantation (1).
Most ST cases were associated with discontinuation of antiplatelet therapy. The discontinuation of antiplatelet medication has been strongly associated with DES thrombosis and is usually seen within two weeks after cessation of antiplatelet treatment, but cases of late ST (LST) or very late ST (VLST) with current antiplatelet medication will lead to a debate on how long one should continue anti-platelet drug therapy after DES implantation.
Here we report a case of VLST at 26 months after DES implantation in a patient with current aspirin and clopidogrel medication.
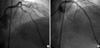
A 73-yr-old female presented to emergency room (ER) in May 2004 with effort angina. She had a history of hypertension. She did not show any specific abnormalities on physical examination. The biochemical test results, including cardiac enzymes, were all normal. Coronary angiography (CAG) revealed a critical lesion in the mid-portion of left anterior descending (LAD) artery (Fig. 1A). She underwent percutaneous coronary intervention (PCI) with one 3.0×33 mm Sirolimus eluting Cypher™ stent (Cordis, Johnson and Johnson, Miami, FL, U.S.A.) for the LAD lesion with acceptable final angiographic results (Fig. 1B). She was discharged with combined anti-platelet agents of aspirin 100 mg and clopidogrel 75 mg, and had uneventful recovery.
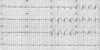
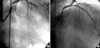
In July 2006, at 26 months after the first PCI, the patient presented to ER with severe chest pain. At the point of time, she did not stop aspirin and clopidogrel medications, nor had any kind of specific medication history. Her electrocardiogram at ER showed 5 mm ST segment elevation with tall T waves in precordial leads suggestive of acute anterior wall myocardial infarction (Fig. 2). The peak level of creatine kinase (CK) was 1,334 U/L, CK-MB was 75.4 U/L, and troponin-I was 38.1 ng/mL. Transthoracic echocardiography revealed anteroseptal wall akinesia and ejection fraction 45%. The patient received a loading dose of 600 mg of clopidogrel and transferred to the cardiac catheterization laboratory. Emergent CAG revealed a thrombotic total occlusion of the middle LAD (Fig. 3A). The occluded LAD was revascularized successfully with balloon angioplasty (Fig. 3B).
Ultegra Rapid Platelet Function Assay (RPFA)-ASA and Ultegra RPFA-P2Y12 (VerifyNow™ Assay®, Accumetrics Inc., San Diego, CA, U.S.A.) were performed to determine aspirin and clopidogrel resistance. The blood sampling was performed 2 weeks after coronary intervention. Aspirin resistance was defined as an aspirin reaction units (ARU) ≥550. Clopidogrel resistance was defined as the percent inhibition of P2Y12 less than 20%. The patient's ARU was 350 and P2Y12 was 90%.
The patient was discharged uneventfully with triple combined anti-platelet regimen of 100 mg aspirin, 75 mg clopidogrel, and 200 mg cilostazol per day after aspirin and clopidogrel resistance test, and has been asymptomatic during the 6-month clinical follow-up.
Nowadays, the introduction of DES has rapidly and profoundly affected the field of interventional cardiology, and DES is used in a majority of intracoronary stenting procedures. DES is successful in a majority of cases in preventing restenosis for the first year following stent implantation.
Although DES has helped overcome the limitation of instent restenosis, a new problem that seems to be associated with the use of DES is ST. ST is a rare, but fatal complication after DES implantation. The recently proposed "Academic Research Consortium" definitions were designed to standardize the diagnosis of ST (2). Acute ST was defined as ST during PCI or within the following 24 hr, subacute ST as between 1 and 30 days following PCI, LST as between 1 month and 1 yr following PCI, and VLST as more than 1 yr following PCI.
In treatment with DES, the rate of acute and subacute ST seems to be similar to that of bare-metal stents (BMS); it remains below 1% for BMS and DES. And the mechanisms underlying the acute and subacute ST after BMS and DES are very similar (3, 4). Ishikawa et al. reported that subacute thrombosis did not increase after stenting with sirolimus eluting Cypher™ stent under combined antiplatelet therapy even in patients with acute myocardial infarction (5).
However, in case of LST or VLST after DES implantation, recent studies have highlighted the increased incidence and potential adverse clinical significance compared with BMS. In the BASKET-LATE study, the rates of LST was higher in DES group (2.6% DES group vs. 1.3% BMS group) and major adverse cardiac events due to LST were also higher in DES group (4.9% DES group vs. 1.3% BMS group, respectively) (6). In a meta-analysis of 9 trials involving 5,261 pateints, increased rates of VLST were found both for SES (0.6% vs. 0%, p=0.025) and PES (0.7% vs. 0.2%, p=0.028), compared with BMS over 4 yr of follow-up (7). Similarly, a meta-analysis of 14 trials on 4,958 patients, with follow-up ranging up to 59 months, showed an increased rate of VLST with SES (0.6% vs. 0.05%, p=0.02) (8).
The reasons for LST or VLST in the population treated with DES remain incompletely understood. The presence of endothelial dysfunction and delayed healing were often described in cases of LST or VLST in DES-treated lesion (9). The mechanism seems to be related to the delay in the healing process and also may be related with late hypersensitivity reaction and consequent inflammatory changes predisposing to stent thrombosis even years after initial deployment. These hypotheses are based upon histological characterization of tissue responses in animal studies revealing arrest of healing process and presence of inflammatory cells as a part of this delayed healing. In addition to this phenomenon, hypersensitivity reaction to the polymer and localized hypersensitivity vasculitis within the stented segment could have contributed to the adverse long-term clinical outcome.
Some clinical features such as premature discontinuation of anti-platelet therapy, diabetes, lower ejection fraction, bifurcation lesions, and stent under-expansion have been identified as independent predictors of VLST (10-12).
In real world, most VLST cases are associated with discontinuation of anti-platelet agent due to dental procedure or non-cardiac surgery. LST or VLST usually occurs within days to months after aspirin and clopidogrel discontinuation, and appears more closely related to discontinuation of aspirin (10, 11).
However, our patient did not have any of the risk factors for VLST; most importantly, he was on current dual antiplatelet therapy for 26 months after PCI and showed no antiplatelet drug resistance by VerifyNow™ Assay® system. A mechanical problem such as an unrecognized edge dissection or under-expansion of stent is unlikely as the time interval was too long. In this case, long lesion was an independent predictive factor of LST or VLST due to delayed endothelialization.
Prolonged combined anti-platelet therapy is needed for such patients at high risk of VLST, but the issue is how to identify the high-risk patients. Perhaps the introduction of new generation of DES with improved biocompatibility and better healing may be one of the answers for the prevention of VLST.
Figures and Tables
Fig. 1
Coronary angiography (CAG) revealed a critical lesion in the middle left anterior descending artery (A). After percutaneous coronary intervention, CAG showed excellent final angiographic results (B).
References
1. Cutlip DE, Baim DS, Ho KK, Popma JJ, Lansky AJ, Cohen DJ, Carrozza JP Jr, Chauhan MS, Rodriguez O, Kuntz RE. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent trials. Circulation. 2001. 103:1967–1971.

2. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007. 115:2344–2351.
3. Moreno R, Fernadez C, Hernadez R, Alfonso F, Angiolillo DJ, Sabaté M, Escaned J, Bañuelos C, Fernández-Ortiz A, Macaya C. Drug-eluting stent thrombosis: results from a pooled analysis including 10 randomized studies. J Am Coll Cardiol. 2005. 45:954–959.
4. Bavry AA, Kumbhani DJ, Helton TJ, Bhatt DL. What is the risk of stent thrombosis associated with the use of paclitaxel-eluting stents for percutaneous coronary intervention?: a meta-analysis. J Am Coll Cardiol. 2005. 45:941–946.

5. Ishikawa T, Mutoh M, Fuda Y, Sakamoto H, Okada H, Higashitani M, Nakano Y, Yamaguchi J, Enta K, Satoh T, Imai K, Horie T, Mochizuki S. Documented subacute stent thrombosis within thirty days after stenting with sirolimus-eluting stent (Cypher) for acute myocardial infarction: a Japanese single center retrospective non-randomized study. Circ J. 2006. 70:1091–1092.
6. Pfisterer M, Brunner-La Rocca HP, Buser PT, Rickenbacher P, Hunziker P, Mueller C, Jeger R, Bader F, Osswald S, Kaiser C. BASKET-LATE Investigators. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006. 48:2584–2591.
7. Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, Colombo A, Schampaert E, Grube E, Kirtane AJ, Cutlip DE, Fahy M, Pocock SJ, Mehran R, Leon MB. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007. 356:998–1008.

8. Kastrati A, Mehilli J, Pache J, Kaiser C, Valgimigli M, Kelbaek H, Menichelli M, Sabate M, Suttorp MJ, Baumgart D, Seyfarth M, Pfisterer ME, Schomig A. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007. 356:1030–1039.

9. Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, Motta T, Mihalcsik L, Tespili M, Valsecchi O, Kolodgie FD. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautions? Circulation. 2004. 109:701–705.
10. Karvouni E, Korovesis S, Katritsis DG. Very late thrombosis after implantation of Sirolimus eluting stent. Heart. 2005. 91:e45.

11. Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005. 293:2126–2130.
12. Ong AT, Hoye A, Aoki J, van Mieghem CA, Rodriguez Granillo GA, Sonnenschein K, Regar E, McFadden EP, Sianos G, van der Giessen WJ, de Jaegere PP, de Feyter P, van Domburg RT, Serruys PW. Thirty-day incidence and six-month clinical outcome of thrombotic stent occlusion after bare-metal, sirolimus, or paclitaxel stent implantation. J Am Coll Cardiol. 2005. 45:947–953.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download