Abstract
Primary non-Hodgkin's lymphoma of bone (PLB) is rare, and generally presents as a single extensive and destructive bone lesion. Histopathologically, most cases present as diffuse large B-cell lymphoma, and T-cell lymphoma is rare. By contrast, multiple myeloma is a disease defined as the neoplastic proliferation of a single clone of plasma cells producing a monoclonal immunoglobulin. We report a case of multiple myeloma that developed during treatment of PLB in a type of T-cell. A 48-yr-old man was diagnosed as T-cell PLB, stage IE, 18 months ago. The patient received the chemoradiotherapy and salvage chemotherapy for PLB. However, the lymphoma progressed with generalized bone pain, and laboratory findings showed bicytopenia and acute renal failure. On bone marrow biopsy, the patient was diagnosed as having multiple myeloma newly developed with primary T-cell lymphoma of bone. In spite of chemotherapy, the patient died of renal failure.
Primary non-Hodgkin's lymphoma of bone (PLB) is a rare disease that accounts for less than 2 percent of all lymphomas in adults (1). The histologies of the vast majority of cases present as diffuse large B-cell lymphoma, while T-cell lymphomas are extremely rare (2-10). Although there are a few publications regarding the characteristics, treatment options, and prognosis of PLB, the optimal management remains unclear. Recently, several reports have supported the combined modality of chemotherapy and radiotherapy (11-13). Multiple myeloma is a B-cell neoplasia that is usually associated with osteolytic lesions and paraprotein in the plasma and/or in the urine (14-16). Because of the uniquely different cell lines of the two lymphoid malignancies, concurrent disease has rarely been reported. To our knowledge, the simultaneous development of primary T-cell lymphoma of bone and multiple myeloma has never been reported. In a view of the same bone lesion in the case of PLB and multiple myeloma, the clinical discrimination of the two diseases is difficult. Through this case, we experienced the importance of reevaluation of disease regarding the possibility of other underlying diseases when it was deteriorated despite adequate treatment.
A 48-yr-old male presented with right ankle pain, mass, and general weakness over 2 months. On physical examination, he appeared acutely ill but no other specific findings were noted.
The complete blood cell counts showed leucocytes 8.7×109/L, hemoglobin 15.5 g/dL, and platelets 245×109/L. Blood chemistry showed total protein 6.6 g/dL, albumin 4.6 g/dL, lactate dehydrogenase 236 U/L, blood urea nitrogen 14.2 mg/dL, and creatine 0.9 mg/dL. Urinalysis was normal. Simple radiography showed an osteolytic lesion in the right distal tibia. Magnetic resonance imaging (MRI) of the right ankle revealed a heterogeneous osteolytic and extraosseous mass in the right tibia (Fig. 1A, B). The bone biopsy showed diffuse infiltration of large tumor cells with vesicular prominent nuclei, abundant cytoplasm, and numerous mitotic figures. Immunohistochemical staining showed an LCA, CD3, CD45RO, and CD15 phenotype (Fig. 2). These findings led to the diagnosis of a T-cell lymphoma. We then performed the staging work-up. Chest and abdomen computed tomography (CT) showed no abnormal findings such as lympadenopathy or hepatosplenomegaly. The Tc99m-methylene diphosphonate (MDP) bone scan revealed hot uptake in the right distal tibia without any other bony involvement (Fig. 1C). The peripheral blood smear was normal, and the bone marrow biopsy showed no lymphoma involvement. The patient was diagnosed as having primary T-cell lymphoma of bone, stage IE. Based on the International Prognostic Index, the patient belonged to a low risk group (17).
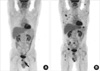
The patient received 6 cycles of CHOP chemotherapy (cyclophosphamide 750 mg/m2 i.v. day 1, doxorubicin 50 mg/m2 i.v. day 1, vincristine 1.4 mg/m2 i.v. day 1, and prednisolone 100 mg p.o. days 1-5). Follow-up MRI showed a partial response. After addition of the involved-field radiation therapy up to 5,700 cGy as once daily fraction and two more cycles of chemotherapy, the tumor mass was further decreased, but still remained on MRI. F-18 fluorodeoxyglucose PET-CT showed the remaining hypermetabolic lesion on tibia, and also revealed hot uptake at the inferior ramus of the right pubis (Fig. 3A). We thought that the disease was progressive and treated the patient with salvage chemotherapy including ICE regimen (ifosfamide 5 g/m2 i.v. day 2, and carboplatin AUC of 5 i.v. day 2, and etoposide 100 mg/m2 i.v. days 1-3) and the DHAP regimen (dexamethasone 40 mg i.v. days 1-4, cytarabine 2 g/m2 i.v. day 2, and cisplatin 100 mg/m2 i.v. D1), but the disease further progressed. The patient received the MINE regimen (ifosfamide 1,333 mg/m2 i.v. days 1-3, mitoxanthrone 8 mg/m2 i.v. day 1, and etoposide 65 mg/m2 i.v. days 1-3) as the fourth line chemotherapy. After three cycles of MINE chemotherapy, restaging work-up showed a partial response. However, at the time of the fourth chemotherapy cycle, he complained of generalized bone pain. Complete blood cell counts revealed bicytopenia (leucocytes 4.5×109/L, hemoglobin 8.8 g/dL, platelets 85×109/L), and the peripheral blood smear showed atypical lymphocytes (2%). Serum creatine was 2.0 mg/dL. Whole body PET revealed multiple hot uptake in the skeletal area (Fig. 3B). Unexpectedly, the bone marrow biopsy showed diffuse infiltration of plasma cells with about 80% of bone marrow cells. Monoclonal gammopathy was detected in urine protein electrophoresis. Urine immunoelectrophoresis identified the monoclonal band as light chain lambda, and the free serum light chain lambda level in urine was 2,810 mg/L (range, 5.71-26.3 mg/L). Bone marrow biopsy showed diffuse uptake of CD138, which was not expressed in the lymphoma mass (Fig. 4). We concluded that the multiple myeloma was newly developed in the patient with primary T-cell lymphoma of bone. The general condition of the patient was rapidly deteriorated with thrombocytopenia and acute renal failure. The patient received a combination chemotherapy with cyclophosphamide and prednisolone, and plasmapheresis was performed to correct paraproteinemia and hypercalcemia. Despite aggressive supportive care, the patient died of renal failure.
PLB generally presents as a solitary, extensive, destructive bone lesion (1). It is a very rare disease that accounts for less than 2 percent of all lymphomas in adults and accounts for approximately 3-5% of all malignant bone tumors and 4-7% of all extranodal non-Hodgkin's lymphomas. Pathologic fracture and systemic 'B' symptoms (fever, weight loss, and night sweats) also develop at the time of diagnosis (4, 5). Most patients suffer from bone pain, which is not relieved by rest. A palpable mass due to soft tissue extension is seen in about one-half of cases (10). It is usually characterized by lytic bones lesions located in the metaphysis of long bones or in the axial skeleton (2-7). Histologic diagnosis may be obtained by percutaneous or open biopsy. Histopathologically, the majority of PLB are diffuse large B-cell type, while T-cell lymphomas are extremely rare (4-7). Management strategies include a combined modality of chemotherapy and radiotherapy (11-13). Although prognosis, survival, and complete remission rates of B-cell PLB were well-described and high complete remission rates have been reported, the treatment response and prognosis of T-cell PLB remain obscure and data are lacking (7-9).
Multiple myeloma is a lymphoproliferative disease as is lymphoma (14). It is characterized by the neoplastic proliferation of a single clone of plasma cells producing a monoclonal immunoglobulin. This clone of plasma cells proliferates in the bone marrow and often results in skeletal osteolytic lesions. Common clinical features include recurrent bacterial infection, anemia, hypercalcemia, and renal insufficiency, and treatments include chemotherapy and radiotherapy (16).
An interesting feature of this case is the development of multiple myeloma over approximately 1.5 yr following the appearance of a primary T-cell lymphoma of bone. He suffered from two lymphoid neoplasms of distinctly different cell lines: the first originating from T-cells and, 1.5 yr later, the second of B-cell origin. Bryant et al. reported plasma cell myeloma in a patient with a cutaneous T-cell lymphoma (17). The investigators assumed that the malignant plasma cells evolved under the sustained inducer stimulus of the neoplastic T-cells. Wickenhauser et al. also reported a newly-detected multiple myeloma in a patient with a long-standing anaplastic cutaneous T-cell lymphoma (20). In the present case, through immunohistochemical CD138 staining, we confirmed that the multiple myeloma originated from cells that differed from previously detected lymphoma cells. Neoplastic T-lymphocytes are known to retain immunoregulatory capabilities similar to those of their normal counterpart (18, 19). We also speculated that the proliferation of T-helper cells might have resulted in a sustained stimulation of B-cells, followed by proliferation of certain B-cell clones. It is also possible, although with a low order of possibility, that the two infrequent malignancies have arisen independently of each other. In this case, it is difficult to determine whether the bone lesion originated from multiple myeloma or lymphoma, based only on imaging studies.
In conclusion, it is important to think about lymphoid malignancies of different pathology when an underlying lymphoid neoplasm shows rapid progression. In addition, considering the scarcity of the association between T-cell lymphoma and multiple myeloma, detection and reporting of new cases are of great value in the study of the potential relationships of immunoregulatory derangements caused by primary lymphoid tumors.
Figures and Tables
Fig. 1
Simple radiography showed osteolytic lesions in the right distal tibia (A). Magnetic resonance imaging (MRI) of the right ankle revealed a heterogeneous, extraosseous mass in the right tibia (B). The Tc-99m bone scan revealed diffuse hot uptake in the right tibia without any other bony involvement at the time of diagnosis of lymphoma (C).
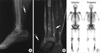
Fig. 2
Diffuse infiltration of large tumor cells with vesicular prominent nucleoli, abundant cytoplasm, and numerous mitotic cells (A, H&E, ×200). Immunohistochemical staining for CD45RO showed positive (B, ×200).

References
1. Dubey P, Ha CS, Besa PC, Fuller L, Cabanillas F, Murray J, Hess MA, Cox JD. Localized primary malignant lymphoma of bone. Int J Radiat Oncol Biol Phys. 1997. 37:1087–1093.

2. Gianelli U, Patriarca C, Moro A, Ponzoni M, Giardini R, Massimino M, Alfano RM, Armiraglio E, Nuciforo P, Bosari S, Coggi G, Parafioriti A. Lymphomas of the bone: a pathological and clinical study of 54 cases. Int J Surg pathol. 2002. 10:257–266.

3. Limb D, Dreghorn C, Murphy JK, Mannion R. Primary lymphoma of bone. Int Orthop. 1994. 18:180–183.

4. Ostrowski ML, Unni KK, Banks PM, Shives TC, Evans RG, O'Connell MJ, Taylor WF. Malignant lymphoma of bone. Cancer. 1986. 58:2646–2655.

6. Zinzani PL, Carrillo G, Ascani S, Barbieri E, Tani M, Paulli M, Stefoni V, Sabattini E, Alinari L, Binazzi R, Tura S, Baccarani M, Pileri SA. Primary bone lymphoma: experience with 52 patients. Haematologica. 2003. 88:280–285.
7. Baar J, Burkes RL, Gospodarowicz M. Primary non-Hodgkin's lymphoma of bone. Semin Oncol. 1999. 26:270–275.
8. Durr HR, Muller PE, Hiller E, Maier M, Baur A, Jansson V, Refior HJ. Malignant lymphoma of bone. Arch Orthop Trauma Surg. 2002. 122:10–16.

9. Heyning FH, Hogendoorn PC, Kramer MH, Hermans J, Kluin-Nelemans JC, Noordijk EM, Kluin PM. Primary non-Hodgkin's lymphoma of bone: a clinicopathological investigation of 60 cases. Leukemia. 1999. 13:2094–2098.

10. Mulligan ME, McRae GA, Murphey MD. Imaging features of primary lymphoma of bone. AJR Am J Roentgenol. 1999. 173:1691–1697.

11. Mendenhall NP, Jones JJ, Kramer BS, Hudson TM, Carter RL, Enneking WF, Marcus RB Jr, Million RR. The management of primary lymphoma of bone. Radiother Oncol. 1987. 9:137–145.

12. Fairbanks RK, Bonner JA, Inwards CY, Strickler JG, Habermann TM, Unni KK, Su J. Treatment of stage IE primary lymphoma of bone. Int J Radiat Oncol Biol Phys. 1994. 28:363–372.

13. Fidias P, Spiro I, Sobczak ML, Nielsen GP, Ruffolo EF, Mankin H, Suit HD, Harmon DC. Long term results of combined modality therapy in primary bone lymphomas. Int J Radiat Oncol Biol phys. 1999. 45:1213–1218.
14. Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson DR, Plevak ME, Therneau TM, Greipp PR. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003. 78:21–33.

15. Lahtinen R, Laakso M, Palva I, Virkkunen P, Elomaa I. Randomised, placebo controlled multicentre trial of clodronate in multiple myeloma. Finnish Leukaemia Group. Lancet. 1992. 340:1049–1052.
16. Blade J, Kyle RA. Multiple myeloma in young patients: Clinical presentation and treatment approach. Leuk Lymphoma. 1998. 30:493–501.
17. A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993. 329:987–994.
18. Bryant E, Ronan SG, Iossifides IA. Plasma cell myeloma in a patient with a cutaneous T-cell lymphoma. Cancer. 1982. 50:2122–2125.

19. Broder S, Poplack D, Whang-Peng J, Durm M, Goldman C, Muul L, Waldmann TA. Characterization of a suppressor cell leukemia: evidence for the requirement of an interaction of two T cells in the development of human suppressor effector cells. N Engl J Med. 1978. 298:66–72.
20. Uchiyama T, Sagawa K, Takatsuki K, Uchino H. Effect of adult T cell leukemia cells on pokeweed mitogen-induced normal B-cell differentiation. Clin Immunol Immunopathol. 1978. 10:24–34.
21. Wickenhauser C, Borchmann P, Diehl V, Scharffetter-Kochanek K. Development of IgG lambda multiple myeloma in a patient with cutaneous CD30+ anaplastic T-cell lymphoma. Leuk Lymphoma. 1999. 35:201–206.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download