Abstract
Although mutations in three genes, amyloid precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2), have been identified as genetic causes of early-onset Alzheimer's disease (EOAD), there has been a single report on a PSEN1 mutation in Koreans. In the present study, we performed a genetic analysis of six Korean patients with EOAD. Direct sequencing analysis of the APP, PSEN1 and PSEN2 genes revealed two different mutations of the PSEN1 gene (G206S and M233T) and one mutation of the APP gene (V715M) in three patients with age-at-onset of 34, 35, and 42 yr, respectively. In addition, two patients with age-at-onset of 55 and 62 yr, respectively, were homozygous for APOE ε4 allele. One woman had no genetic alterations. These findings suggest that PSEN1 and APP gene mutations may not be uncommon in Korean patients with EOAD and that genetic analysis should be provided to EOAD patients not only for the identification of their genetic causes but also for the appropriate genetic counseling.
Alzheimer's disease (AD) is a progressive neurodegenerative disease that is characterized by memory loss and personality changes. The age of onset in AD may vary widely and this is the basis for the classification into early- and late-onset, with 60 or 65 yr being the usual cutoff point (1, 2). There are currently three known causative genes in early-onset AD (EOAD): the amyloid precursor protein gene (APP) on chromosome 21 at 21q21.1 (3); the presenilin-1 gene (PSEN1) on chromosome 14 at 14q24.3 (4); and the presenilin-2 gene (PSEN2) on chromosome 1 at 1q42.1 (5, 6). In addition, the apolipoprotein E (APOE) ε4 allele has been reported to be a susceptibility gene for the development of familial and/or sporadic early- and late-onset AD (7-9).
Mutations in the PSEN1 gene account for 18-55% of familial EOAD (1, 10). PSEN2 mutations are much rarer causes of familial EOAD with 19 families being reported, including the Volga-German kindred where a founder effect was demonstrated (1, 2, 5, 6, 11). Twenty-seven different APP mutations have been reported in 74 families and all mutations are clustered in or adjacent to the amyloid β (Aβ) peptide sequence, the major component of the amyloid plaques (12). PSEN1 mutations, along with APP mutations, are believed to be pathogenic by altering APP processing to change the Aβ40:Aβ42 ratio (13, 14).
In the Korean population, there has been only a single report on a mutation in the PSEN1 gene in a pedigree of a 36-yr-old familial AD (15). In this study, mutation analysis of the APP, PSEN1, and PSEN2 genes was performed to determine the contribution of these genes in the genetic background of EOAD patients in Korea.
Six patients were evaluated by a neurologist and were diagnosed of having AD according to the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association (NINCDSADRDA) criteria (16). Computed tomography (CT), magnetic resonance image (MRI), and/or positron emission tomography (PET) were performed to rule out other causes of dementia. A family history was obtained from the patients or their relatives.
After obtaining informed consent, screening for mutations in the APP, PSEN1, and PSEN2 genes were performed in the patients with EOAD. All coding exons and their flanking intronic sequences were analyzed for PSEN1 and PSEN2 genes but selected exons (exons 16 and 17) were tested for APP. Genomic DNA was extracted from peripheral blood leukocytes using a Wizard Genomic DNA Purification Kit according to the manufacturer's instructions (Promega, Madison, WI, U.S.A.). Each exon was amplified by polymerase chain reaction (PCR) using the primers designed by the authors (available on request). Direct sequencing was performed using a BigDye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems, Foster City, CA, U.S.A.) on an ABI Prism 3100 genetic analyzer (Applied Biosystems). APOE genotyping was performed with a commercial kit using the multiplex amplification refractory mutation system (Bio-Core ApoE Kit, Bio-Core, Seoul, Korea).
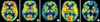
Table 1 gives a summary of clinical findings of the six patients with EOAD. The mean age of onset was 43.6 yr (range, 34 to 62 yr), and all patients except patient 3 had a family history of dementia (Fig. 1). All the patients showed progressive impairment of their episodic memory. Patient 1 was diagnosed as definite AD by a neuropathology study that showed neuritic plaque, amyloid deposits and neurofibrillary tangles in the brain. PET imaging of the glucose metabolism in all patients except patient 4 revealed severe hypometabolism in all patients (Fig. 2). An EEG study was done in patients 1 and 2 and mild to moderate diffuse cerebral dysfunction was observed.
Two different mutations of the PSEN1 gene were detected (G206S and M233T) in patients 3 and 2 with an age of onset of 34 and 35 yr, respectively. In the APP gene, one mutation was detected (V715M) in patient 1 with the age of onset of 41 yr. All were missense mutations and have been described previously. PSEN2 gene analysis was performed in three patients without a mutation within either PSEN1 or APP, but no mutation was detected. Two of them carried the APOE ε4/ε4 allele, and their age of onset was relatively late (62 and 55 yr) compared with the mutation positive patients and had affected families with late-onset AD. Patient 6 did not have any mutation. After identification of PSEN1 and APP mutations in the patients, we tried to perform genetic analysis in the family members of the patients but failed due to either absence of living affected relatives or refusal of genetic analysis.
In this study, we found two mutations in the PSEN1 gene, G206S and M233T, and one mutation in the APP gene, V715M, in three unrelated Korean patients. The V715M mutation in the APP gene is the first mutation identified in this gene in Korean patients with EOAD. This mutation was previously detected in a French family and the age of onset of the proband in the French family was 41 yr, which is same as the onset age in our patient (10). In addition, the other family members (two paternal uncles) developed dementia at ages 52 and 60, respectively. However, the family members of our patient (the father and one paternal uncle) developed AD earlier at ages 45 and around the 5th decade, respectively.
The G206S mutation in the PSEN1 gene has been reported in two families (17, 18), and their age of onset was 30 to 35 yr, which is similar to that in our patients. The M233T mutation was previously identified in French and Australian families with 3-5 affected individuals in each family and the mean age of onset of 35 yr (10, 17, 19). And, it is of note that our patient showed rapidly progressive course by worsening the Korean Mini-Mental State Examination (K-MMSE) score from 20 at 2 yr after the first symptom into 4 at 2 yr later, which might be due to the presence of the APOE ε4 allele.
No mutations in the PSEN1, PSEN2, and APP genes were found in 3 patients. Interestingly, the age of onset in these patients was more than 20 yr later (55-62 yr old) than those with identified mutations (34-42 yr old). They had a family history of late-onset AD as well. Two of the three patients were homozygous carriers for APOE ε4 allele that could be classified into the AD type 2 (MIM 104310). In a Korean population, it is reported that the frequency of APOE ε4 allele is 0.09-0.128 (20-22) and that of genotype ε4/ε4 was as low as 0.006-0.009 (20, 21, 23).
Up to now, 27 mutations of the APP gene, 159 mutations in the PSEN1 gene, and 11 mutations in the PSEN2 gene have been reported in EOAD worldwide (www.molgen.ua.ac.be/ADMutations). In previous reports, mutational analysis of three genes in 96 autosomal dominant EOAD families led to the conclusion that 77% of cases in these families could be attributed to mutations within the PSEN1 and APP genes (10, 17, 24).
In Korea, there was only one report of genetically confirmed case of EOAD (15). However, although the number of study subjects was small, this study shows that PSEN1 or APP gene mutations exist in other Korean patients with EOAD and few reports on the EOAD patients confirmed by genetic analysis might be due to under-utilization of genetic tests. Therefore, we suggest that screening for PSEN1 and APP gene mutations should be provided in Korean patients with EOAD, especially in patients with age of onset <55, not only for the molecular diagnosis of the patients but also for the appropriate genetic counseling of the patients and their family members. In addition, APOE genotype might be also important to rule out familial AD in Koreans.
Figures and Tables
Fig. 1
Pedigrees of Korean patients with EOAD. Numbers below symbols are age-at-onset and age-at-deceased, respectively.
Circle, female; square, male; filled symbol, affected; open symbol, not affected; open symbol with a question mark, affected status not known; question mark, age-at-onset not known; horizontal bar, alive.
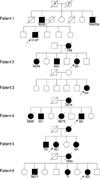
Fig. 2
Comparison of brain FDG-PET images from a cognitively normal control and four patients with EOAD. FDG-PET images (B-E) show variable degrees of hypometabolism in temporoparietal and frontal cortex, which are typical for Alzheimer's disease. (A) a 55-yr-old healthy Korean with a K-MMSE score of 30, (B) patient 2; 3 yr after the first symptom with a K-MMSE score of 22, (C) patient 3; 4 yr after the first symptom with a K-MMSE score of 4, (D) patient 5; 3 yr after the first symptom with a K-MMSE score of 18; (E) patient 6; 3 yr after the first symptom with a K-MMSE score of 11.
References
1. Cruts M, van Duijn CM, Backhovens H, Van den Broeck M, Wehnert A, Serneels S, Sherrington R, Hutton M, Hardy J, St George-Hyslop PH, Hofman A, Van Broeckhoven C. Estimation of the genetic contribution of presenilin-1 and -2 mutations in a population-based study of presenile Alzheimer disease. Hum Mol Genet. 1998. 7:43–51.

2. Finckh U, Muller-Thomsen T, Mann U, Eggers C, Marksteiner J, Meins W, Binetti G, Alberici A, Hock C, Nitsch RM, Gal A. High prevalence of pathogenic mutations in patients with early-onset dementia detected by sequence analyses of four different genes. Am J Hum Genet. 2000. 66:110–117.

3. Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, Mant R, Newton P, Rooke K, Roques P, Talbot C, Pericak-Vance M, Roses A, Williamson R, Rossor M, Owen M, Hardy J. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991. 349:704–706.

4. Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin JF, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HAR, Haines JL, Pericak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St George-Hyslop PH. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995. 375:754–760.

5. Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T, Mar L, Sorbi S, Nacmias B, Placentini S, Amaducci L, Chumakov I, Cohen D, Lannfelt L, Fraser PE, Rommens JM, St George-Hyslop PH. Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature. 1995. 376:775–778.

6. Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, Crowley AC, Fu YH, Guenette SY, Galas D, Nemens E, Wijsman EM, Bird TD, Schellenberg GD, Tanzi RE. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995. 269:973–977.

7. Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993. 261:921–923.

8. Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, Hulette C, Crain B, Goldgaber D, Roses AD. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993. 43:1467–1472.
9. van Duijn CM, de Knijff P, Cruts M, Wehnert A, Havekes LM, Hofman A, Van Broeckhoven C. Apolipoprotein E4 allele in a population-based study of early-onset Alzheimer's disease. Nat Genet. 1994. 7:74–78.

10. Campion D, Dumanchin C, Hannequin D, Dubois B, Belliard S, Puel M, Thomas-Anterion C, Michon A, Martin C, Charbonnier F, Raux G, Camuzat A, Penet C, Mesnage V, Martinez M, Clerget-Darpoux F, Brice A, Frebourg T. Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am J Hum Genet. 1999. 65:664–670.

11. Lao JI, Beyer K, Fernandez-Novoa L, Cacabelos R. A novel mutation in the predicted TM2 domain of the presenilin 2 gene in a Spanish patient with late-onset Alzheimer's disease. Neurogenetics. 1998. 1:293–296.

12. Esler WP, Wolfe MS. A portrait of Alzheimer secretases--new features and familiar faces. Science. 2001. 293:1449–1454.

13. Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996. 2:864–870.
14. Ancolio K, Dumanchin C, Barelli H, Warter JM, Brice A, Campion D, Frebourg T, Checler F. Unusual phenotypic alteration of beta amyloid precursor protein (betaAPP) maturation by a new Val-715 --> Met betaAPP-770 mutation responsible for probable early-onset Alzheimer's disease. Proc Natl Acad Sci USA. 1999. 96:4119–4124.
15. Hong KS, Kim SP, Na DL, Kim JG, Suh YL, Kim SE, Kim JW. Clinical and genetic analysis of a pedigree of a thirty-six-year-old familial Alzheimer's disease patient. Biol Psychiatry. 1997. 42:1172–1176.

16. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDSADRDA Work Group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 1984. 34:939–944.

17. Raux G, Guyant-Marechal L, Martin C, Bou J, Penet C, Brice A, Hannequin D, Frebourg T, Campion D. Molecular diagnosis of autosomal dominant early onset Alzheimer's disease: an update. J Med Genet. 2005. 42:793–795.
18. Rogaeva EA, Fafel KC, Song YQ, Medeiros H, Sato C, Liang Y, Richard E, Rogaev EI, Frommelt P, Sadovnick AD, Meschino W, Rockwood K, Boss MA, Mayeux R, St George-Hyslop P. Screening for PS1 mutations in a referral-based series of AD cases: 21 novel mutations. Neurology. 2001. 57:621–625.

19. Kwok JB, Taddei K, Hallupp M, Fisher C, Brooks WS, Broe GA, Hardy J, Fulham MJ, Nicholson GA, Stell R, St George Hyslop PH, Fraser PE, Kakulas B, Clarnette R, Relkin N, Gandy SE, Schofield PR, Martins RN. Two novel (M233T and R278T) presenilin-1 mutations in early-onset Alzheimer's disease pedigrees and preliminary evidence for association of presenilin-1 mutations with a novel phenotype. Neuroreport. 1997. 8:1537–1542.
20. Kang SY, Lee WI. Apolipoprotein E polymorphism in ischemic stroke patients with different pathogenetic origins. Korean J Lab Med. 2006. 26:210–216.

21. Kim JH, Lim HS, Kwon OH. Polymorphisms of apolipoprotein B and apolipoprotein E in hypobetalipoproteinemic Korean. Korean J Lab Med. 2002. 22:388–394.
22. Ki CS, Na DL, Kim HJ, Kim JW. Alpha-1 antichymotrypsin and alpha-2 macroglobulin gene polymorphisms are not associated with Korean late-onset Alzheimer's disease. Neurosci Lett. 2001. 302:69–72.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download