Abstract
Clear cell tumor of the lung is a rare and very unusual benign pulmonary tumor. As clear cell tumor of the lung contains abundant cytoplasmic glycogen, this tumor is called "sugar tumor". We report a case of sugar tumor in a 64-yr-old man presenting as a round pulmonary nodule. On dynamic computed tomography (CT) scans, the solitary pulmonary nodule showed early wash-in enhancement with an early washout pattern like a lung malignancy. The patient underwent wedge resection for the tumor. Pathologic examination, including immunohistochemical studies, revealed that the nodule was a benign clear cell tumor, so-called "sugar tumor". Because only a small number of cases have been reported previously, clinical aspects, radiological characteristics on dynamic contrast-enhanced CT, and differential diagnosis of the tumor are not well established. Herein we present a clear cell tumor of the lung and discuss the clinical, radiological, and pathological features of the tumor.
Clear cell "sugar" tumor of the lung is a rare benign neoplasm, initially described by Liebow and Castleman in 1963 (1, 2). The tumor has been usually presented as an isolated and asymptomatic pulmonary nodule on chest radiogram (3). The sugar tumor may occur in any lobe and is mainly located under the pleura with no communication with bronchi (4, 5). Equally often affecting both sexes, the tumor occurs in various age groups, but is most often seen in the elderly (6). The tumor is composed of clear cells with large amounts of cytoplasmic periodic acid-Schiff (PAS)-positive glycogen; therefore, this tumor is called clear cell tumor or sugar cell tumor. The tumor cells show immunoreactivity for S-100 protein and human melanoma black (HMB)-45 and no reactivity for cytokeratin, which usually establishes the definitive diagnosis (7). S-100 protein is normally present in cells derived form the neural crest, chondrocytes, adipocytes, myoepithelial cells, macropharges, Langerhans cells, dendritic cells, and keratinocytes. This protein family is useful as markers for certain tumors including melanomas, peripheral nerve sheath tumors, and clear cell tumors and epidermal differentiation. HMB-45 is a monoclonal antibody that reacts against an antigen present in melanocytic tumors and also specific for clear cell tumors. The sugar tumor is invariably benign and surgical resection is curative. Although these characteristics of this tumor have been well defined, only sporadic cases of this neoplasm have been reported in the literature (8). Moreover, the radiological features of the tumor on dynamic contrast enhanced computed tomography (CT) including wash-in and washout patterns have not been released.
In this report, we present a case of the clear cell tumor, the "sugar" tumor, in the lung of a 64-yr-old man with its clinical, radiological, and pathologic features.
A 64-yr-old man was admitted to the hospital because of an abnormal shadow, about 1-cm sized solitary pulmonary nodule (SPN), on chest radiography. He was diagnosed as chronic obstructive pulmonary disease (Global Initiative for Chronic Obstructive Lung Disease criteria class II) in 1998 and had taken medical treatment for the disorder. He was a 30 pack-year smoker.
Chest radiographs showed a round, smooth-margined SPN in the left upper lobe (Fig. 1A). Chest CT showed an SPN measuring 12×11×11 mm in the anterior segment of left upper lobe (Fig. 1B). Dynamic contrast-enhanced CT scans also revealed that the SPN was well enhanced above 60 Hounsfield Unit (HU) in early phase and showed an early washout pattern (Fig. 1C).
The patient underwent a left thoracotomy and a wedge resection was performed for the pulmonary tumor with diagnostic and curative purpose. The tumor was well-circumscribed, grayish-white on cut surface and measured 12×10 mm in diameter (Fig. 2). There was no necrosis or hemorrhage. The tumor was not encapsulated but it was relatively easy to separate from the surrounding pulmonary parenchyma.
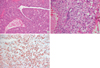
Histologically, the tumor consisted of sheet of neoplastic cells surrounded by thin-walled blood vessels with various sizes. Relatively numerous clefts resembling bronchiole or metaplastic alveoli were recognized (Fig. 3A). The vasculature was prominent and often tumor cells were arranged in a radial fashion around blood vessels. However, there was no vessel with a thick muscular wall. The tumor cells were polygonal with abundant clear to eosinophilic granular cytoplasm and distinct cytoplasmic membranes (Fig. 3B). Few mitotic figures were recognized in 50 high power fields (HPF). The clear cytoplasm contained numerous glycogen granules as demonstrated by PAS staining. The immunohistochemical studies showed strong immunoreactivity for HMB-45 in most tumor cells (Fig. 3C) and some cells showed a positive reaction to S-100 protein, but no reactivity for cytokeratin. The diagnosis of the benign clear cell sugar tumor of the lung was established. For exclusion of the metastatic renal cell carcinoma, abdominal, and pelvic CT was performed and showed no abnormal findings.
No postoperative complications occurred. At 2-month follow-up, he had no complaints and no evidence for the disease was found.
The primary clear cell tumor of the lung is an extremely rare benign tumor, which is called "sugar tumor", because of the large content of glycogen (4). Approximately 40 cases of sugar tumor in the lung have been reported (9). Patients usually range in age from 40 to 60 yr with equal sex prevalence. The typical clinical manifestation is incidentaloma on routine chest radiographs or CT scans. Most patients with the tumor are asymptomatic, except for a few cases of symptoms such as bloody sputum or hemoptysis (9). Benign clear cell sugar tumor is very often misdiagnosed as a pulmonary metastasis of clear cell renal carcinoma (8, 10). In some instances, melanoma metastasizing to the lung or a primary clear cell carcinoma of the lung is also considered (8). Thus, the differential diagnosis is critically important for the appropriate management for the patients, although they are rare clinical entities.
Radiographically, benign clear cell sugar tumor presents as a rounded, smooth-walled, and peripheral parenchymal nodule without evidence of cavitation or calcification. There are no specific lobar distributions (9). One of the characteristics on contrast-enhanced CT scan for the sugar tumor is the intense post-contrast enhancement (11) that appears to be related to its rich vascular stroma as described in rounded atelectasis in workers exposed to asbestos (12). In general, malignant nodules tend to be enhanced substantially more than benign nodules do (13-15). However, in cases of active granulomas or benign vascular tumors, it is difficult to differentiate them from malignant nodules using only early-phase dynamic CT scan (14, 15). A recent study has demonstrated that the pattern of wash-in and washout on dynamic contrast-enhanced CT is helpful for the differentiation between malignant and benign nodules and that malignant nodules can be characterized by means of a net enhancement of more than 25 HU or more and a washout of 5-31 HU (16). In the case of our patient, dynamic contrast-enhanced chest CT scan revealed a well-enhanced nodule (above 60 HU) in early phase with an early washout pattern on the anterior segment of left upper lobe. These patterns indicate the malignant potential of this nodule rather than a benign nodule. Therefore, the sugar tumor can be misdiagnosed as a malignant tumor on dynamic contrast chest CT scan as in cases of active tuberculoma or sclerosing hemangioma.
Macroscopically, benign clear cell sugar tumor predominantly appears as well-circumscribed and peripheral nodules measuring less than 3 cm in diameter (9). Cut surfaces are typically homogeneous and glistening without evidence of hemorrhage, necrosis, cavitation, or calcification. Histologically, tumor cells are typical large cells with clear abundant cytoplasm and neither atypia nor mitoses, consistent with the usual behavior of a benign tumor (17). In the present case, the tumor cells showed typical morphologic features of clear cell sugar tumor with few mitoses in 50 HPF and no necrosis.
Clear cell sugar tumor is characterized immunohistochemically by immunoreactivity for HMB-45 and S-100 protein (18-20), and no reactivity for cytokeratin or epithelial membrane antigen (EMA). Our present case showed the typical immunohistochemical findings for clear cell sugar tumor: positive for HMB-45 and S-100 protein and negative for cytokeratin.
While the histogenesis of clear cell sugar tumor is unclear, a hypothesis that has been most extensively investigated is that, like lymphangioleiomyomatosis and angiolipoma, it originates from perivascular epitheloid cells. These tumors show HMB-45 reactivity, antibodies to perivascular myoid cell proliferation, and melanosomes, all of which are suggestive of pericyte origin (17, 21).
The findings of a clear cell tumor calls for differential diagnosis between a benign tumor and clear cell pulmonary carcinoma, which is characterized by nuclear pleomorphism, abundant mitosis, necrosis, immunohistochemical reactivity for cytokeratin and EMA, and the ultrastructural presence of zymogen granules (21). A second differential diagnosis to be considered is renal carcinoma metastasis, which presents intracytoplasmic accumulations of glycogen and lipids as well as immunohistochemical reactivity for cytokeratin and EMA (18).
On the basis of histological and immunohistological findings, we could exclude clear cell pulmonary carcinoma and renal carcinoma metastasis and the diagnosis of benign sugar tumor in the lung was established. From a review of the literature, we found that certain clinicopathologic features, such as a diameter greater than 2.5 cm, the presence of symptoms, and extensive necrosis or abundant mitoses, visible under an optical microscope, are associated with more aggressive behavior (17, 18, 21). Using only cytologic study such as fine needle aspiration or biopsy, it is difficult to know these clinicopathological features of the lesion. Therefore, the surgical excision of the nodules, especially in suspected the sugar tumor, which is both diagnostic and curative, seems to be the best solution.
Figures and Tables
Fig. 1
Chest radiography (A) revealed an SPN in the left upper lobe. The dynamic contrast-enhanced chest CT (B) showed a well-enhanced SPN measuring 12×11×11 mm on anterior segment of left upper lobe in early phase with an early washout enhancement pattern (C). Each arrow in panel A and B indicates SPN.
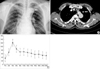
Fig. 2
Macroscopic finding of the tumor. The tumor sized 12×10 mm was resected and easily enucleated. The tumor was nonencapsulated, well circumscribed, and grayish-white on cut surface.
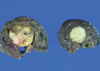
Fig. 3
Representative hematoxylin and eosin stained sections of the lung mass at low magnification (A) and at high magnification (B). The tumor cells arranged in sheets or trabuculae separated by various sized thin-walled blood vessels. In addition, the tumor cells were polygonal with abundant clear to eosinophilic cytoplasm and distinct cytoplasmic membranes. In the immunohistochemical studies, the tumor cells showed strong immunoreactivity for HMB-45 (C).
References
1. Liebow AA, Castleman B. Benign "clear cell tumors" of the lung. Am J Pathol. 1963. 43:13–14.
2. Liebow AA, Castleman B. Benign clear cell ("sugar") tumors of the lung. Yale J Biol Med. 1971. 43:213–222.
3. Shimosato Y, Miller RR. Biopsy interpretation of the lung. 1995. New York: Raven Press.
4. Andrion A, Mazzucco G, Gugliotta P, Monga G. Benign clear cell (sugar) tumor of the lung: a light microscopic, histochemical, and ultrastructural study with a review of the literature. Cancer. 1985. 56:2657–2663.

5. Ozdemir IA, Zaman NU, Rullis I, Webb WR. Benign clear cell tumor of lung. J Thorac Cardiovasc Surg. 1974. 68:131–133.
6. Papla B, Demczuk S, Malinowski E. Benign clear-cell "sugar" tumor of the lung-a case report. Pol J Pathol. 2003. 54:183–185.
7. Gaffey MJ, Mills SE, Zarbo RJ, Weiss LM, Gown AM. Clear cell tumor of the lung. Immunohistochemical and ultrastructural evidence of melanogenesis. Am J Surg Pathol. 1991. 15:644–653.

8. Dail DH. Dail DH, Hammar SP, Colby TV, editors. Uncommon tumors. Pulmonary Pathology Tumors. 1995. New York: Springer;219–224.

9. Santana AN, Nunes FS, Ho N, Takagaki TY. A rare cause of hemoptysis: benign sugar (clear) cell tumor of the lung. Eur J Cardiothorac Surg. 2004. 25:652–654.

10. Gal AA, Koss MN, Hochholzer L, Chejfec G. An immunohistochemical study of benign clear cell ("sugar") tumor of the lung. Arch Pathol Lab Med. 1991. 115:1034–1038.
11. Seo JB, Im JG, Seo JW, Yeon KM. Clear cell tumor of the lung. AJR Am J Roentgenol. 1996. 166:730–731.

12. Terra-Filho M, Kavakama J, Bagatin E, Capelozzi VL, Nery LE, Tavares R. Identification of rounded atelectasis in workers exposed to asbestos by contrast helical computed tomography. Braz J Med Biol Res. 2003. 36:1341–1347.

13. Swensen SJ, Brown LR, Colby TV, Weaver AL, Midthun DE. Lung nodule enhancement at CT: prospective findings. Radiology. 1996. 201:447–455.

14. Yi CA, Lee KS, Kim EA, Han J, Kim H, Kwon OJ, Jeong YJ, Kim S. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology. 2004. 233:191–199.

15. Zhang M, Kono M. Solitary pulmonary nodules: evaluation of blood flow patterns with dynamic CT. Radiology. 1997. 205:471–478.

16. Jeong YJ, Lee KS, Jeong SY, Chung MJ, Shim SS, Kim H, Kwon OJ, Kim S. Solitary pulmonary nodule: characterization with combined wash-in and washout features at dynamic multi-detector row CT. Radiology. 2005. 237:675–683.

17. Jordá Aragón C, Froufe Sánchez A, Padilla Alarcón J. Benign clear cell tumor of the lung. Arch Bronconeumol. 2005. 41:59.

18. Gaffey MJ, Mills SE, Askin FB, Ross GW, Sale GE, Kulander BG, Visscher DW, Yousem SA, Colby TV. Clear cell tumor of the lung. A clinicopathologic, immunohistochemical, and ultrastructural study of eight cases. Am J Surg Pathol. 1990. 14:248–259.

19. Gaffey MJ, Mills SE, Ritter JH. Clear cell tumors of the lower respiratory tract. Semin Diagn Pathol. 1997. 14:222–232.
20. Gaffey MJ, Mills SE, Frierson HF Jr, Askin FB, Maygarden SJ. Pulmonary clear cell carcinoid tumor: another entity in the differential diagnosis of pulmonary clear cell neoplasia. Am J Surg Pathol. 1998. 22:1020–1025.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download