Abstract
Co-infection of herpes simplex virus type 1 (HSV-1) and human cytomegalovirus (HCMV) is not uncommon in immunocompromised hosts. Importantly, organ transplant recipients concurrently infected with HSV-1 and HCMV have a worse clinical outcome than recipients infected with a single virus. However, factors regulating the pathologic response in HSV-1, HCMV co-infected tissues are unclear. We investigated the potential biologic role of HCMV gene product immediate early 1 (IE1) protein in HSV-1-induced syncytial formation in U373MG cells. We utilized a co-infection model by infecting HSV-1 to U373MG cells constitutively expressing HCMV IE1 protein, UMG1-2. Syncytial formation was assessed by enumerating nuclei number per syncytium and number of syncytia. HSV-1-induced syncytial formation was enhanced after 24 hr in UMG1-2 cells compared with U373MG controls. The amplified phenotype in UMG1-2 cells was effectively suppressed by roscovitine in addition to inhibitors of viral replication. This is the first study to provide histological evidence of the contribution of HCMV IE1 protein to enhanced cytopathogenic responses in active HSV-1 infection.
Herpes simplex virus type 1 (HSV-1) is a member of the herpesvirsus family, which infection commonly involves recurrent mucocutaneous manifestations (1). HSV-1 could also cause illness in a much severe form; ocular herpes could cause adult blindness, furthermore induce sporadic encephalitis, usually resulting in severe morbidity or mortality (2). Moreover, HSV-1 is one of the leading infectious viral pathogens found in immunocompromised hosts, such as transplant recipients. HSV-1-induced tissue damage comprises of mononuclear cell infiltration, perivascular inflammation, as well as various cytopathogenic changes including intracellular inclusion bodies and syncytial formation (3). Especially, syncytial formation is a characteristic pathologic response seen in herpesvirus or human immunodeficiency virus type 1 (HIV-1) infections (3, 4).
This fusogenic activity in HSV-1-infected cells requires the presence of specific glycoproteins in the virion envelope, including glycoprotein B (gB), gD, gH, and gL (5-8). Syncytial formation starts with the binding of virus-infected cells to uninfected cells through viral glycoprotein-receptor interaction, followed by cell-to-cell fusion to form a multinucleated giant cell, the syncytium. This eventually ruptures to cause cell death. It is noteworthy that patients infected with syncytium-inducing (SI) HIV-1 variants have a more rapid deterioration in their course than those infected with non-SI variants (9, 10).
In immunocompromised hosts infected with HSV-1, concurrent infections by other microorganisms, including other viruses are not uncommon. Human cytomegalovirus (HCMV) is another important causal agent in opportunistic infection, which can induce comparable cytopathogenic effects in infected cells (11). There have been numerous reports of co-infection of HSV-1 and HCMV in immunocompromised patients (12-14). The clinical outcome of herpesvirus co-infection is most appreciated in follow-up studies after transplantation. For example, grafts infected with both HSV-1 and HCMV results in shorter graft and patient survival than grafts with either single viral infection (14). However, there are no study yet which investigates the pathology in HSV-1, HCMV co-infected tissue or organs.
We hypothesized that in the setting of active HSV-1 and HCMV co-infection, HCMV could exacerbate HSV-1-induced cytopathogenic changes in HSV-1 permissive cells. In particular, we focused on the HCMV gene products from the major immediate early (IE) gene. By utilizing a U373MG cell line expressing HCMV IE1 protein (15), we investigated the function of HCMV IE1 protein in modulating HSV-1 induced syncytial formation.
We utilized cell line UMG1-2 that constitutively expresses HCMV IE1 protein in U373MG cells (ATCC HTB 17) (15). U373MG cells transfected with the empty retrovirus vector LNCX2 vector (Clontech, Palo Alto, CA, U.S.A.) was used as the control. Cells were cultured in Dulbecco's modified Eagles medium (GIBCO, Grand Island, NY, U.S.A.) with 10% fetal bovine serum (GIBCO), 100 U/mL penicillin and 100 U/mL streptomycin (Life Technologies, Carlsbad, CA, U.S.A.) in a 37℃ incubator with 5% CO2. HSV-1 MacIntyre (ATCC VR-539) was used in the experiment.
Neutralizing anti-HSV-1 monoclonal antibody, MHSVI116 (2 µg/mL) was used as previously described (16). Other reagents were used in the following concentration: ganciclovir (40 µM), mitomycin C (1 µg/mL), nocodazole (10 µg/mL), N-tosyl-1-phenylalanine-chloromethyl ketone (TPCK) (10, 100 µM), emodin (1, 10 µM) and roscovitine (2-100 µM). All reagents were purchased from Sigma (St. Louis, MO, U.S.A.). Concentrations of the drugs were determined by appraisal of former reports of drug treatment in U373MG cells or in studies utilizing HSV-1 (15, 17-19). Drugs were prepared in 100× concentrations.
Ten thousand cells were plated on each compartment of an 8-chamber slide (Sigma) and cultured for 24 hr. HSV-1 stock (2.0×106 plaque forming unit/mL) was diluted 10 fold from 10-1 to 10-4 in volume. UMG1-2 and its control in each compartment were infected with 100 µL of HSV-1 for 1 hr followed by washing phosphate-buffered saline (PBS) and addition of 100 µL of media. Slides were fixed with 4% paraformaldehyde-PBS after 6, 12, 24, 48, and 72 hr and stained with hematoxylin & eosin. The size of each syncytium was evaluated by visible nuclei numbers per syncytium under a light microscope (8). Syncytium with 5 or more nuclei was enumerated in five low-power fields. Experiments were repeated three times.
Cells were infected with 0.2 multiplicity of infection (m.o.i.) of HSV-1 for 1 hr and were added with media after washing with PBS. Immunohistochemistry was done with the following protocol; cells were fixed with 4% paraformaldehyde-PBS for 1 hr and then permeabilized with 0.5% Nonidet P-40 (Sigma)-PBS for 5 min. After washing, wells were blocked with 2% skim milk for 30 min, and then treated with MHSVI116 antibody for 1 hr at room temperature. Peroxidase-conjugated anti-mouse IgG (Jackson Immunoresearch, West Grove, PA, U.S.A.) in 2% skim milk was added for 1 hr at room temperature. Finally, wells were treated with 0.2% diaminobenzidine (Sigma) and 0.005% hydrogen peroxide in PBS for 20 min. Reaction was stopped with distilled water and the plates were observed under an inverted microscope.
Cells were concomitantly treated with the drug of interest with HSV-1 infection (0.2 m.o.i.). Culture media with or without dimethyl sulfoxide (DMSO, Sigma) was used as controls. After infecting cells with HSV-1 for 1 hr, cells were washed with PBS, and then each drug concentration was maintained for 24 hr. Cells were fixed and immunohistochemistry was performed as described, followed by enumeration of syncytia (nuclei ≥5) under an inverted microscope. Experiments were repeated three times.
Both UMG1-2 and U373MG control cells formed syncytia after HSV-1 infection. Syncytial formation was negligible at 6 hr post-infection even in high HSV-1 concentrations but noticeable at 12 hr at 2 m.o.i. (data not shown). At 24 hr, we could appreciate a clear m.o.i.-dependent syncytial formation by HSV-1 (Fig. 1A). The syncytial formation effect was enhanced in UMG1-2 cells compared with the U373MG control, especially the size of the syncytium. The nuclei number per syncytium was significantly higher in UMG1-2 cells than in U373MG control cells (Fig. 1B). The number of syncytia in the two groups was not different at 2 m.o.i., as all sizes of syncytia were included. With ongoing fusogenic activity in both groups of cells, syncytia enumeration was difficult in longer periods of infection (48, 72 hr) due to cell lysis, especially in UMG1-2 cells in which cell lysis was detected earlier, at a lower m.o.i. due to robust syncytial formation (data not shown).
For further characterization of the enhanced cytopathogenic changes in UMG1-2 cells, cells were treated with blocking antibody or inhibitors pertinent to viral multiplication and intracellular signaling associated with herpesvirus infection (Fig. 2A). First, MHSVI116, a neutralizing antibody against HSV-1 gB, completely abrogated syncytial formation by HSV-1 in both UMG1-2 and U373MG controls. Nocodazole was used to impair microtubule-mediated transport of HSV-1 capsid to the nucleus (20). It markedly suppressed syncytial formation in both cell lines as expected. Syncytial formation was also compromised in both cell lines by blocking viral DNA synthesis with ganciclovir or mitomycin C. After treatment of cyclin-dependent kinase (cdk) inhibitor roscovitine, both UMG1-2 and U373MG controls showed decreased syncytial formation (Fig. 2B). Importantly, the enhanced cytopathogenic response in UMG1-2 was diminished by roscovitine. Moreover, nuclei numbers per syncytium were significantly lower in both cell lines treated with roscovitine (media vs. roscovitine-treated UMG1-2 cells ([mean±SD] 27.4±5.8 vs. 12.1±3.5, p=0.001). Other inhibitors such as tyrosine kinase inhibitor, emodin, and NF-κB inhibitor, TPCK, did not suppress syncytial formation in either cell lines after infection.
To further investigate the dose response of roscovitine in HSV-1-infected cells, we next compared syncytial formation in different concentrations of roscovitine (Fig. 3). Roscovitine inhibited syncytial formation in both HSV-1-infected cells in a dose-dependent manner at 24 hr post-infection. With increase concentration of roscovitine, enhanced syncytial formation in HSV-1-infected UMG1-2 cells was effectively neutralized.
HSV-1 infection with co-infection with other viruses, could cause severe illness in immunocompromised hosts (14). By utilizing a HSV-1 permissive U373MG cell line constitutively expressing HCMV IE1 protein, we demonstrated enhanced syncytial formation in IE1 protein expressing UMG1-2 cells after HSV-1 infection. Furthermore, we showed that along with potent viral replication inhibitors and neutralizing antibodies, roscovitine could effectively inhibit the augmented the cytopathogenic effect. To better control the variability produced by the complex biology of co-infection models, we postulated that HCMV major IE gene products could be a major factor modulating HSV-1 pathogenecity in host cells co-infected with both herpesviruses. This hypothesis was supported by in vitro studies demonstrating HSV-2 reactivation by HCMV in the very early stages of HCMV super-infection (21, 22).
HCMV IE1 protein, one of the two (IE1 and IE2) transcription factors of the major IE promoter, is the most abundantly expressed viral protein in the immediate early phase of HCMV-infected permissive human cell lines (23, 24). HCMV IE1 protein is expressed shortly after HCMV infection and its level increases steadily through 72 hr (25). Moreover, HCMV infection induces dominant expression of IE1 protein compared with IE2 protein in many cancer cell lines such as Saos-2 and U373MG (15). In fact, HCMV IE1 protein helps expression of viral and cellular promoters synergistically with IE2 protein (26). Interactions have been reported between HCMV IE1 protein and other transcription factors, such as SP-1 and CTF-1, or the TFIID complex (27-29). Especially, HCMV IE1-TFIID interaction leads to inhibition of apoptosis induced by tumor necrosis factor in vitro (30, 31).
The functional role of HCMV IE1 protein in host cell growth has been previously described in several transfection studies (32-35). HCMV IE1 protein was first known to interact with retinoblastoma susceptibility gene-related p107 protein to overcome p107-induced cellular growth arrest (33, 34). Subsequent studies revealed that HCMV IE1 but not IE2 protein, could bind to p107 and facilitate cyclinE/cdk2 activation (35). Furthermore, HCMV IE1 protein induces resistance to apoptosis in U373MG cells by cdk2 accumulation (15). Cdks are required for replication of many viruses, including HIV-1, HSV-2, HCMV, and varicella-zoster virus (36-40). Among pharmacological cdk inhibitors, roscovitine has already been investigated as a potential anticancer drug for disrupting the cell cycle (41). The emerging interest in roscovitine is its antiviral effect, such that it specifically inhibits genes in viruses such as HIV-1, HSV-2, HCMV, and varicella-zoster virus (42, 43). In HSV-1-infected cells, roscovitine blocks accumulation of mRNAs encoding specific viral IE and early genes, inhibits viral DNA synthesis, and alters posttranslational modification of HSV-1 IE protein (38, 44-47). In general, roscovitine is likely to inhibit HSV-1-induced syncytial formation by interfering viral replication. Considering the significant reduction in syncytial size, we further speculate roscovitine may also have affected late expression of viral proteins, including viral glycoproteins necessary for syncytial formation.
Inhibitors of NF-κB, and tyrosine kinsase we used did not affect syncytial formation in HCMV IE1 protein expressing cells. Interestingly, previous studies showed that HCMV IE1 protein selectively induce both NF-κB complexes and its activity (48-50). In addition, HSV-1 infection also results in intranuclear translocation of NF-κB (51). However, our results show that NF-κB signaling is not the major pathway that HCMV IE1 protein contributes to the phenotype of enhanced syncytial formation by HSV-1. As for the tyrosine kinase inhibitor, emodin, we were unable to detect any evidence of emodin affecting syncytial formation, in spite of reported antiviral effects (19, 52).
There are yet limitations to indicate HCMV IE1 protein to be the essential molecule in vivo modulating increased cytopathogenecity in HCMV, HSV-1 co-infected cells. Our model is based on the constitutive expression of HCMV IE1 protein in host cells, disregarding the life cycle and other gene products of HCMV. Furthermore, previous in vitro studies demonstrate functional conservation in viral gene products of both HCMV and HSV-1 (53-55). Thus, it is possible that other HCMV gene products could also potentiate cytopathogenicity induced by HSV-1, especially by facilitating viral replication (56). Further investigations will include identifying genes modulated by HCMV IE1 in the syncytium, especially genes in viral glycoprotein synthesis.
In summary, this is the first study to implicate the potential contribution of HCMV IE1 protein to enhance cytopathogenic responses in active HSV-1 infection. In addition to known potent inhibitors of viral replication, roscovitine was able to effectively suppress HCMV IE1 protein-induced cytopathologies, supporting its effective anti-viral activity.
Figures and Tables
Fig. 1
Enhanced syncytial formation in HSV-1-infected UMG1-2 cells. (A) UMG1-2 and U373MG control cells were infected with HSV-1 for 24 hr on a chamber slide. Note the increased syncytial formation in UMG1-2 cells compared with U373MG controls (100× magnification). (B) Enumeration of nuclei per syncytium and number of syncytia after 24 hr. Note the increased syncytial formation, especially nuclei number of syncytium in UMG1-2 cells (*p<0.05, †p<0.01, ‡p<0.001). Values are expressed as mean±standard error and represent data from 3 independent experiments.
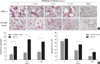
Fig. 2
Roscovitine ameliorates augmented syncytial formation in HSV-1-infected UMG1-2 cells. (A) Cells were plated on a 96-well plate and treated with described reagents simultaneously at the time of HSV-1 infection for 24 hr. MHSVI-116, ganciclovir, mitomycin C, and nocodazole robustly suppressed syncytial formation of both groups of cells. Roscovitine (20 µM) also effectively inhibited syncytial formation in UMG1-2 cells as well as U373MG control cells (*p<0.05, †p<0.01). TPCK, N-tosyl-1-phenylalanine-chloromethyl ketone. Values are expressed as mean±standard error and represent data from 3 independent experiments. (B) Comparison of immunohistochemistry results of roscovitine vs. media-only treated wells 24 hr after HSV-1 infection.

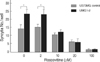
Fig. 3
Dose-dependent inhibition of syncytial formation by roscovitine. Syncytial formation in both groups of cells are suppressed with an increasing dose of roscovitine. Note the enhanced syncytial formation in UMG1-2 cells is suppressed to the level of U373MG controls at concentrations 20 µM or more (*p<0.05). Values are expressed as mean±standard error and represent data from 3 independent experiments.
References
1. Corey L, Spear PG. Infections with herpes simplex viruses (1). N Engl J Med. 1986. 314:686–691.
2. Najioullah F, Bosshard S, Thouvenot D, Boibieux A, Menager B, Biron F, Aymard M, Lina B. Diagnosis and surveillance of herpes simplex virus infection of the central nervous system. J Med Virol. 2000. 61:468–473.

3. Ecob-Johnston MS, Whetsell WO Jr. Host-cell response to herpes virus infection in central and peripheral nervous tissue in vitro. J Gen Virol. 1979. 44:747–757.

4. Yoffe B, Lewis DE, Petrie BL, Noonan CA, Melnick JL, Hollinger FB. Fusion as a mediator of cytolysis in mixtures of uninfected CD4+ lymphocytes and cells infected by human immunodeficiency virus. Proc Natl Acad Sci USA. 1987. 84:1429–1433.

5. Stegmann T, Doms RW, Helenius A. Protein-mediated membrane fusion. Annu Rev Biophys Biophys Chem. 1989. 18:187–211.

7. Browne H, Bruun B, Minson T. Plasma membrane requirements for cell fusion induced by herpes simplex virus type 1 glycoproteins gB, gD, gH and gL. J Gen Virol. 2001. 82:1419–1422.

8. Turner A, Bruun B, Minson T, Browne H. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J Virol. 1998. 72:873–875.

9. Koot M, Keet IP, Vos AH, de Goede RE, Roos MT, Coutinho RA, Miedema F, Schellekens PT, Tersmette M. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993. 118:681–688.

10. Katzenstein TL. Molecular biological assessment methods and understanding the course of the HIV infection. APMIS Suppl. 2003. 1–37.
12. Laskin OL, Stahl-Bayliss CM, Morgello S. Concomitant herpes simplex virus type 1 and cytomegalovirus ventriculoencephalitis in acquired immunodeficiency syndrome. Arch Neurol. 1987. 44:843–847.

13. Vital C, Monlun E, Vital A, Martin-Negrier ML, Cales V, Leger F, Longy-Boursier M, Le Bras M, Bloch B. Concurrent herpes simplex type 1 necrotizing encephalitis, cytomegalovirus ventriculoencephalitis and cerebral lymphoma in an AIDS patient. Acta Neuropathol (Berl). 1995. 89:105–108.

14. Dunn DL, Matas AJ, Fryd DS, Simmons RL, Najarian JS. Association of concurrent herpes simplex virus and cytomegalovirus with detrimental effects after renal transplantation. Arch Surg. 1984. 119:812–817.

15. Kim J, Kwon YJ, Park ES, Sung B, Kim JH, Park CG, Hwang ES, Cha CY. Human cytomegalovirus (HCMV) IE1 plays role in resistance to apoptosis with etoposide in cancer cell line by Cdk2 accumulation. Microbiol Immunol. 2003. 47:959–967.

16. Cha CY, Hwang ES, Kook YH. Production and characterization of monoclonal antibodies specific to herpes simplex viruses. J Korean Soc Microbiol. 1988. 23:505–515.
17. Mahboobi S, Pongratz H, Hufsky H, Hockemeyer J, Frieser M, Lyssenko A, Paper DH, Burgermeister J, Bohmer FD, Fiebig HH, Burger AM, Baasner S, Beckers T. Synthetic 2-aroylindole derivatives as a new class of potent tubulin-inhibitory, antimitotic agents. J Med Chem. 2001. 44:4535–4553.

18. Wing BA, Johnson RA, Huang ES. Identification of positive and negative regulatory regions involved in regulating expression of the human cytomegalovirus UL94 late promoter: role of IE2-86 and cellular p53 in mediating negative regulatory function. J Virol. 1998. 72:1814–1825.

19. Sydiskis RJ, Owen DG, Lohr JL, Rosler KH, Blomster RN. Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob Agents Chemother. 1991. 35:2463–2466.

20. Sodeik B, Ebersold MW, Helenius A. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997. 136:1007–1021.

21. Colberg-Poley AM, Isom HC, Rapp F. Reactivation of herpes simplex virus type 2 from a quiescent state by human cytomegalovirus. Proc Natl Acad Sci USA. 1979. 76:5948–5951.

22. Colberg-Poley AM, Isom HC, Rapp F. Involvement of an early human cytomegalovirus function in reactivation of quiescent herpes simplex virus type 2. J Virol. 1981. 37:1051–1059.

23. Wathen MW, Stinski MF. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J Virol. 1982. 41:462–477.

24. Wilkinson GW, Kelly C, Sinclair JH, Rickards C. Disruption of PML-associated nuclear bodies mediated by the human cytomegalovirus major immediate early gene product. J Gen Virol. 1998. 79(Pt 5):1233–1245.

25. Stenberg RM, Depto AS, Fortney J, Nelson JA. Regulated expression of early and late RNAs and proteins from the human cytomegalovirus immediate-early gene region. J Virol. 1989. 63:2699–2708.

26. Malone CL, Vesole DH, Stinski MF. Transactivation of a human cytomegalovirus early promoter by gene products from the immediate-early gene IE2 and augmentation by IE1: mutational analysis of the viral proteins. J Virol. 1990. 64:1498–1506.

27. Luu P, Flores O. Binding of SP1 to the immediate-early protein-responsive element of the human cytomegalovirus DNA polymerase promoter. J Virol. 1997. 71:6683–6691.

28. Hayhurst GP, Bryant LA, Caswell RC, Walker SM, Sinclair JH. CCAAT box-dependent activation of the TATA-less human DNA polymerase alpha promoter by the human cytomegalovirus 72-kilodalton major immediate-early protein. J Virol. 1995. 69:182–188.

29. Lukac DM, Harel NY, Tanese N, Alwine JC. TAF-like functions of human cytomegalovirus immediate-early proteins. J Virol. 1997. 71:7227–7239.

30. Lafemina RL, Pizzorno MC, Mosca JD, Hayward GS. Expression of the acidic nuclear immediate-early protein (IE1) of human cytomegalovirus in stable cell lines and its preferential association with metaphase chromosomes. Virology. 1989. 172:584–600.

31. Zhu H, Shen Y, Shenk T. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J Virol. 1995. 69:7960–7970.

32. Pajovic S, Wong EL, Black AR, Azizkhan JC. Identification of a viral kinase that phosphorylates specific E2Fs and pocket proteins. Mol Cell Biol. 1997. 17:6459–6464.

33. Poma EE, Kowalik TF, Zhu L, Sinclair JH, Huang ES. The human cytomegalovirus IE1-72 protein interacts with the cellular p107 protein and relieves p107-mediated transcriptional repression of an E2F-responsive promoter. J Virol. 1996. 70:7867–7877.

34. Johnson RA, Yurochko AD, Poma EE, Zhu L, Huang ES. Domain mapping of the human cytomegalovirus IE1-72 and cellular p107 protein-protein interaction and the possible functional consequences. J Gen Virol. 1999. 80:1293–1303.

35. Zhang Z, Huong SM, Wang X, Huang DY, Huang ES. Interactions between human cytomegalovirus IE1-72 and cellular p107: functional domains and mechanisms of up-regulation of cyclin E/cdk2 kinase activity. J Virol. 2003. 77:12660–12670.

36. Sanchez V, McElroy AK, Yen J, Tamrakar S, Clark CL, Schwartz RA, Spector DH. Cyclin-dependent kinase activity is required at early times for accurate processing and accumulation of the human cytomegalovirus UL122-123 and UL37 immediate-early transcripts and at later times for virus production. J Virol. 2004. 78:11219–11232.

37. Sanchez V, Spector DH. Cyclin-dependent kinase activity is required for efficient expression and posttranslational modification of human cytomegalovirus proteins and for production of extracellular particles. J Virol. 2006. 80:5886–5896.

38. Schang LM, Phillips J, Schaffer PA. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J Virol. 1998. 72:5626–5637.

39. Hossain A, Holt T, Ciacci-Zanella J, Jones C. Analysis of cyclin-dependent kinase activity after herpes simplex virus type 2 infection. J Gen Virol. 1997. 78:3341–3348.

40. Habran L, Bontems S, Di Valentin E, Sadzot-Delvaux C, Piette J. Varicella-zoster virus IE63 protein phosphorylation by roscovitine-sensitive cyclin-dependent kinases modulates its cellular localization and activity. J Biol Chem. 2005. 280:29135–29143.

41. Benson C, White J, De Bono J, O'Donnell A, Raynaud F, Cruickshank C, McGrath H, Walton M, Workman P, Kaye S, Cassidy J, Gianella-Borradori A, Judson I, Twelves C. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br J Cancer. 2007. 96:29–37.

42. De Azevedo WF, Leclerc S, Meijer L, Havlicek L, Strnad M, Kim SH. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. Eur J Biochem. 1997. 243:518–526.

43. Schang LM. Cyclin-dependent kinases as cellular targets for antiviral drugs. J Antimicrob Chemother. 2002. 50:779–792.

44. Schang LM, Rosenberg A, Schaffer PA. Transcription of herpes simplex virus immediate-early and early genes is inhibited by roscovitine, an inhibitor specific for cellular cyclin-dependent kinases. J Virol. 1999. 73:2161–2172.

45. Schang LM, Rosenberg A, Schaffer PA. Roscovitine, a specific inhibitor of cellular cyclin-dependent kinases, inhibits herpes simplex virus DNA synthesis in the presence of viral early proteins. J Virol. 2000. 74:2107–2120.

46. Davido DJ, Leib DA, Schaffer PA. The cyclin-dependent kinase inhibitor roscovitine inhibits the transactivating activity and alters the posttranslational modification of herpes simplex virus type 1 ICP0. J Virol. 2002. 76:1077–1088.

47. Diwan P, Lacasse JJ, Schang LM. Roscovitine inhibits activation of promoters in herpes simplex virus type 1 genomes independently of promoter-specific factors. J Virol. 2004. 78:9352–9365.

48. Sambucetti LC, Cherrington JM, Wilkinson GW, Mocarski ES. NF-kappa B activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. Embo J. 1989. 8:4251–4258.

49. Jiang HY, Petrovas C, Sonenshein GE. RelB-p50 NF-kappa B complexes are selectively induced by cytomegalovirus immediate-early protein 1: differential regulation of Bcl-x(L) promoter activity by NF-kappa B family members. J Virol. 2002. 76:5737–5747.
50. Kim S, Yu SS, Kim VN. Essential role of NF-kappa B in transactivation of the human immunodeficiency virus long terminal repeat by the human cytomegalovirus 1E1 protein. J Gen Virol. 1996. 77:83–91.
51. Patel A, Hanson J, McLean TI, Olgiate J, Hilton M, Miller WE, Bachenheimer SL. Herpes simplex type 1 induction of persistent NF-kappa B nuclear translocation increases the efficiency of virus replication. Virology. 1998. 247:212–222.
52. Andersen DO, Weber ND, Wood SG, Hughes BG, Murray BK, North JA. In vitro virucidal activity of selected anthraquinones and anthraquinone derivatives. Antiviral Res. 1991. 16:185–196.

53. Gao M, Robertson BJ, McCann PJ, O'Boyle DR, Weller SK, Newcomb WW, Brown JC, Weinheimer SP. Functional conservations of the alkaline nuclease of herpes simplex type 1 and human cytomegalovirus. Virology. 1998. 249:460–470.

54. Reid GG, Ellsmore V, Stow ND. An analysis of the requirements for human cytomegalovirus oriLyt-dependent DNA synthesis in the presence of the herpes simplex virus type 1 replication fork proteins. Virology. 2003. 308:303–316.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download